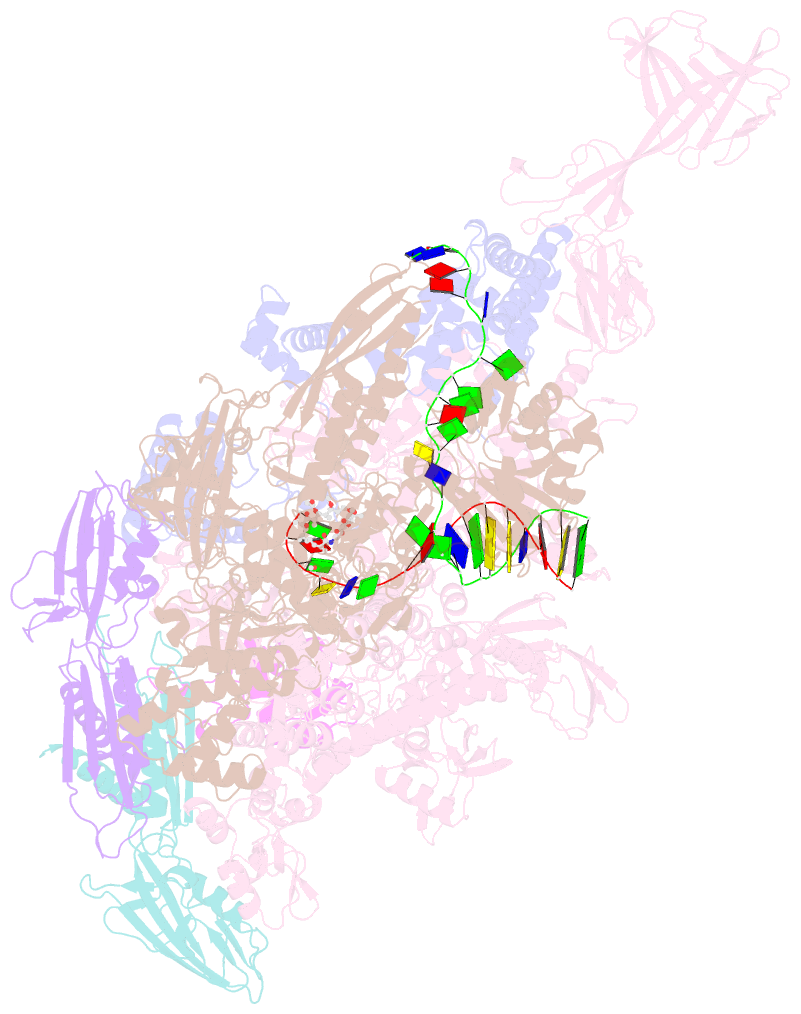
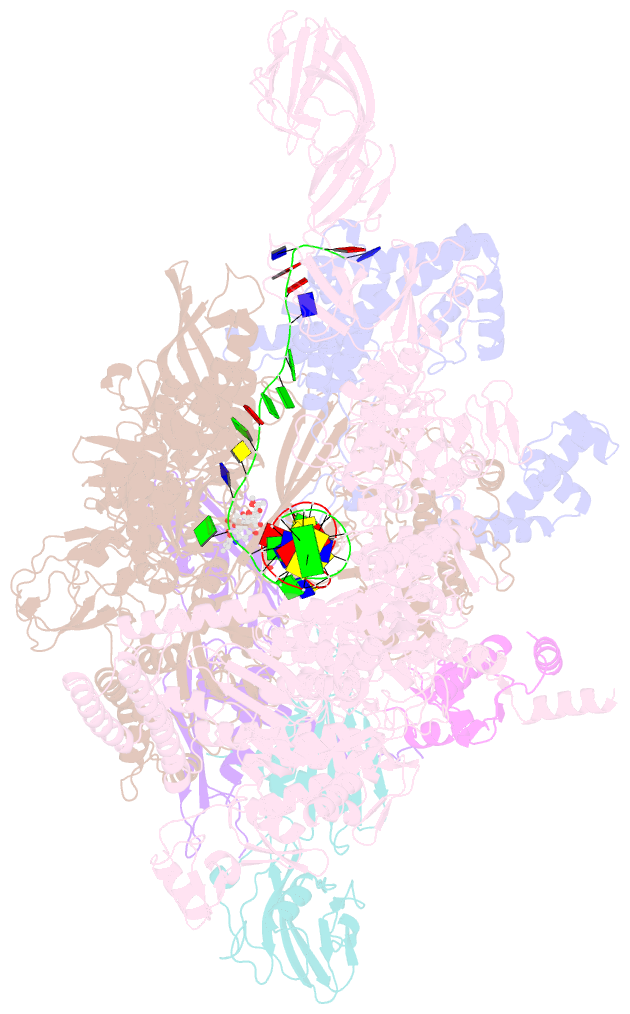
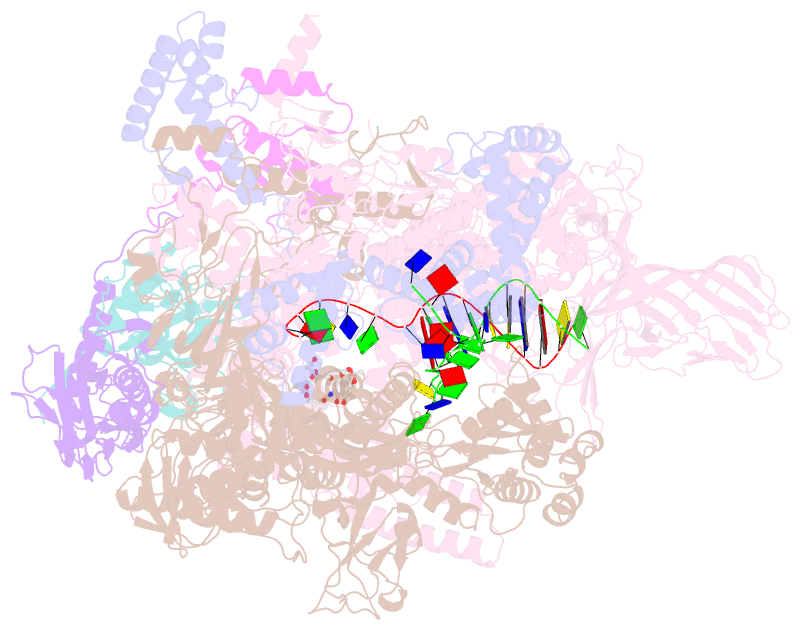
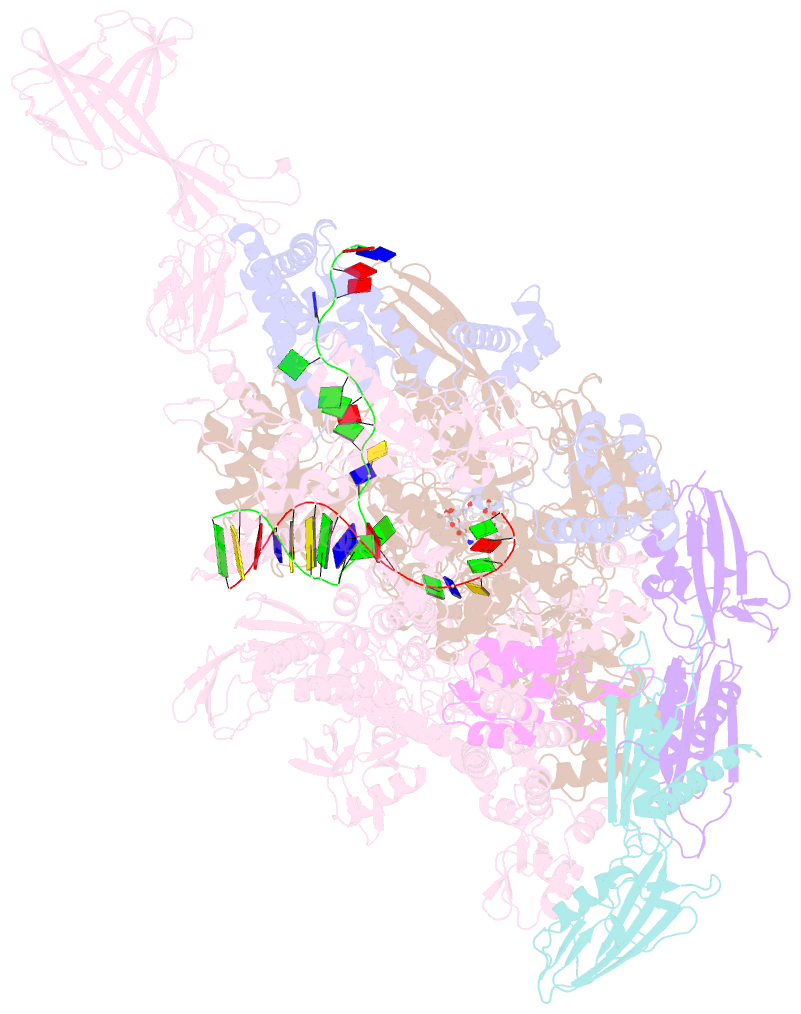
Summary information and primary citation
- PDB-id
- 4oir; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription, transferase-antibiotic
- Method
- X-ray (3.105 Å)
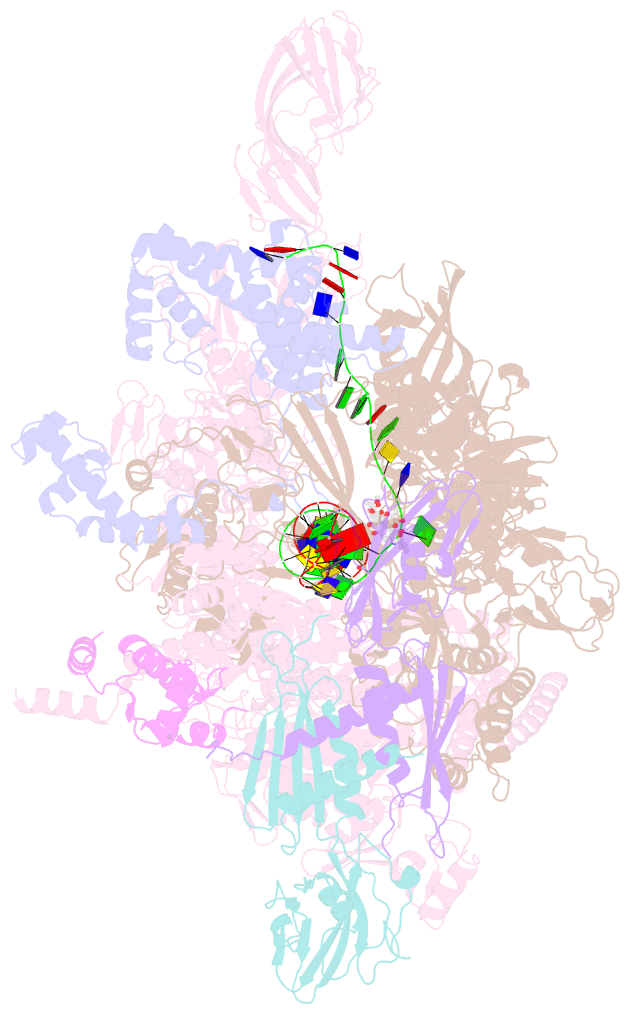
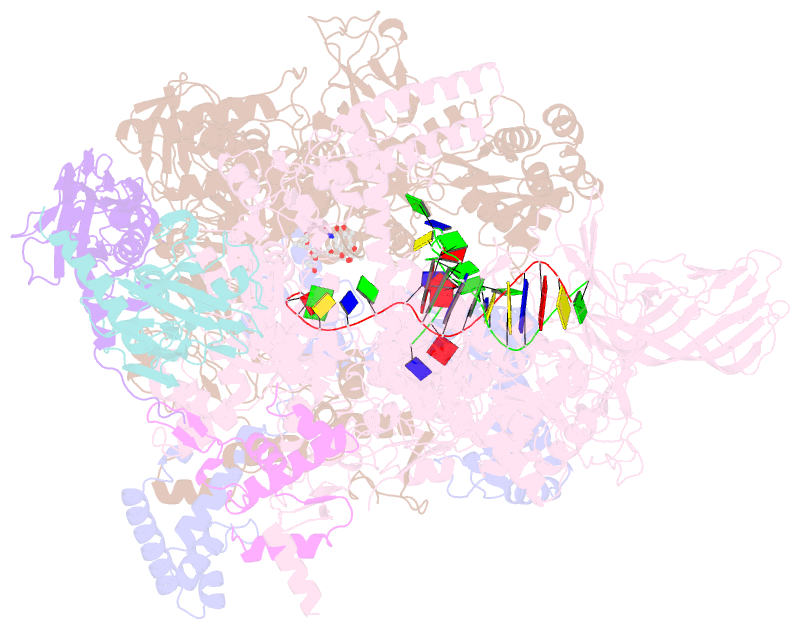
- Summary
- Crystal structure of thermus thermophilus RNA polymerase transcription initiation complex soaked with ge23077 and rifamycin sv
- Reference
- Zhang Y, Degen D, Ho MX, Sineva E, Ebright KY, Ebright YW, Mekler V, Vahedian-Movahed H, Feng Y, Yin R, Tuske S, Irschik H, Jansen R, Maffioli S, Donadio S, Arnold E, Ebright RH (2014): "GE23077 binds to the RNA polymerase 'i' and 'i+1' sites and prevents the binding of initiating nucleotides." Elife, 3, e02450.
- Abstract
- Using a combination of genetic, biochemical, and structural approaches, we show that the cyclic-peptide antibiotic GE23077 (GE) binds directly to the bacterial RNA polymerase (RNAP) active-center 'i' and 'i+1' nucleotide binding sites, preventing the binding of initiating nucleotides, and thereby preventing transcription initiation. The target-based resistance spectrum for GE is unusually small, reflecting the fact that the GE binding site on RNAP includes residues of the RNAP active center that cannot be substituted without loss of RNAP activity. The GE binding site on RNAP is different from the rifamycin binding site. Accordingly, GE and rifamycins do not exhibit cross-resistance, and GE and a rifamycin can bind simultaneously to RNAP. The GE binding site on RNAP is immediately adjacent to the rifamycin binding site. Accordingly, covalent linkage of GE to a rifamycin provides a bipartite inhibitor having very high potency and very low susceptibility to target-based resistance. DOI: http://dx.doi.org/10.7554/eLife.02450.001.