Summary information and primary citation
- PDB-id
- 4ola; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-RNA
- Method
- X-ray (2.3 Å)
- Summary
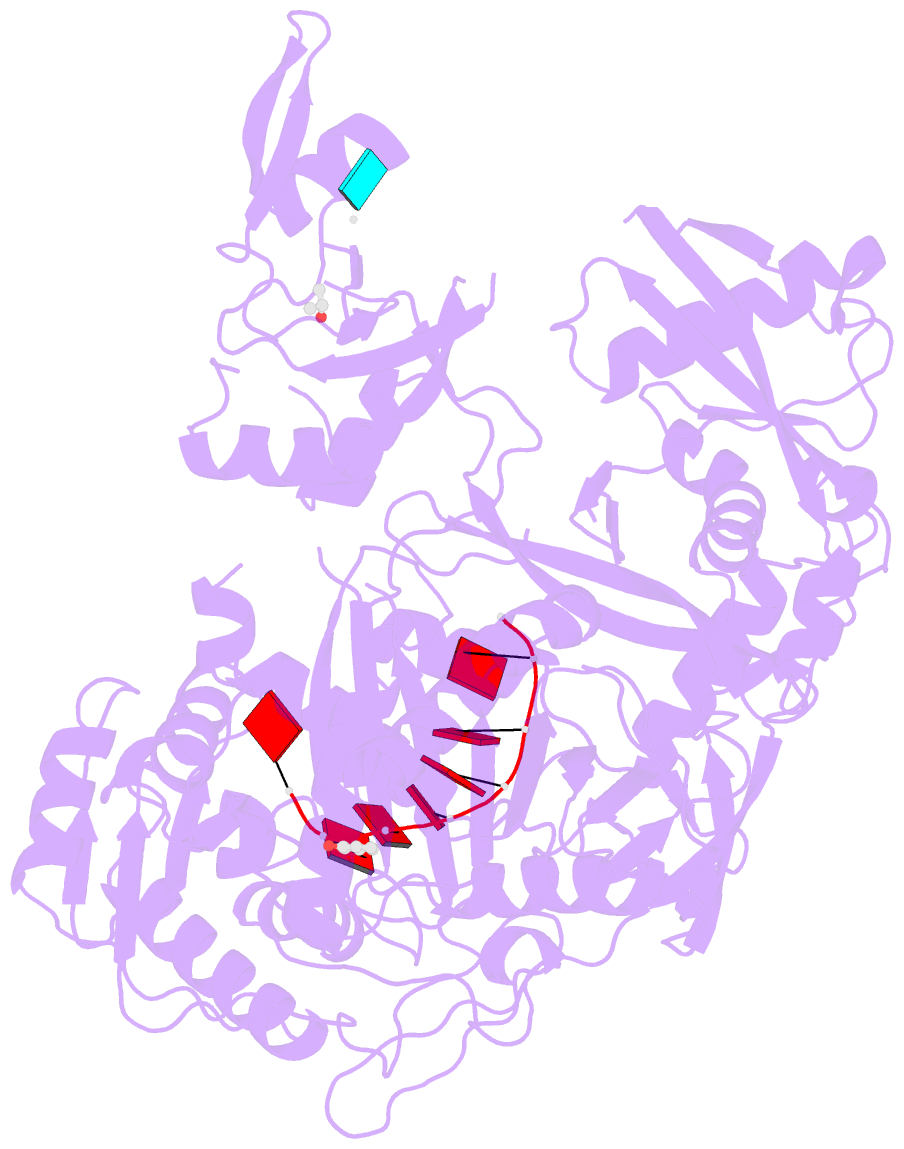
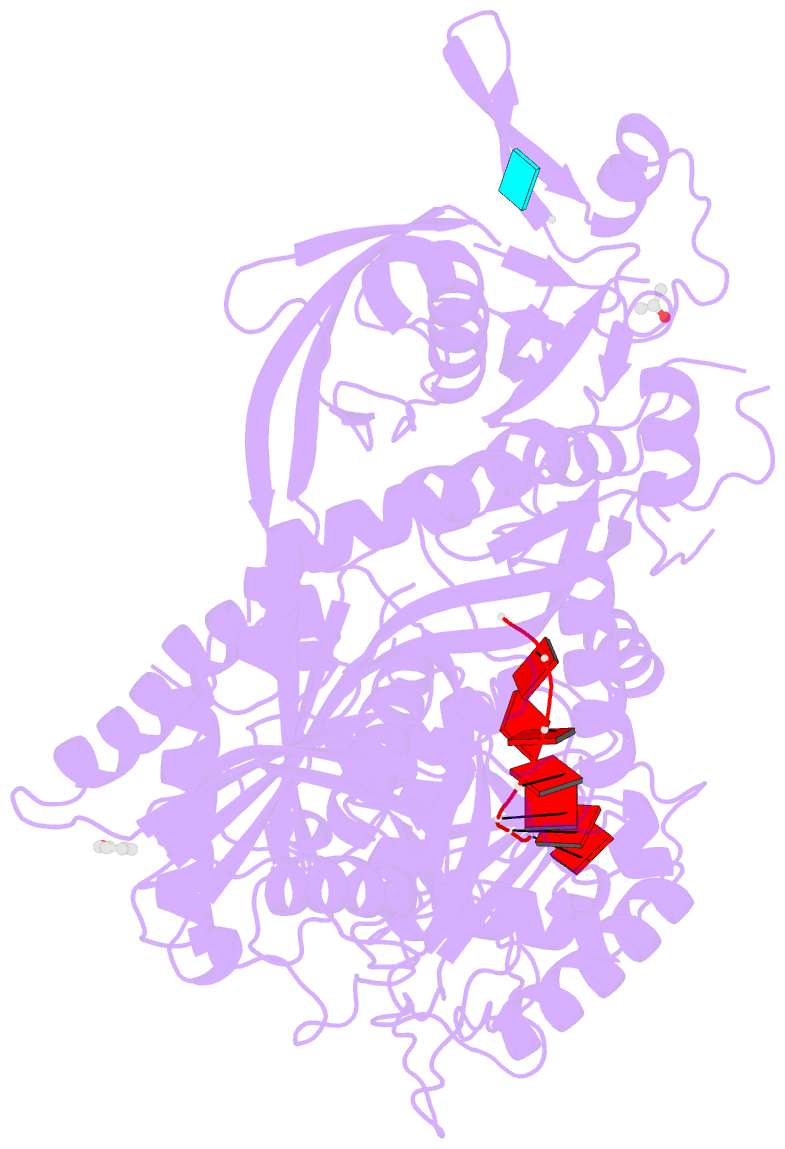
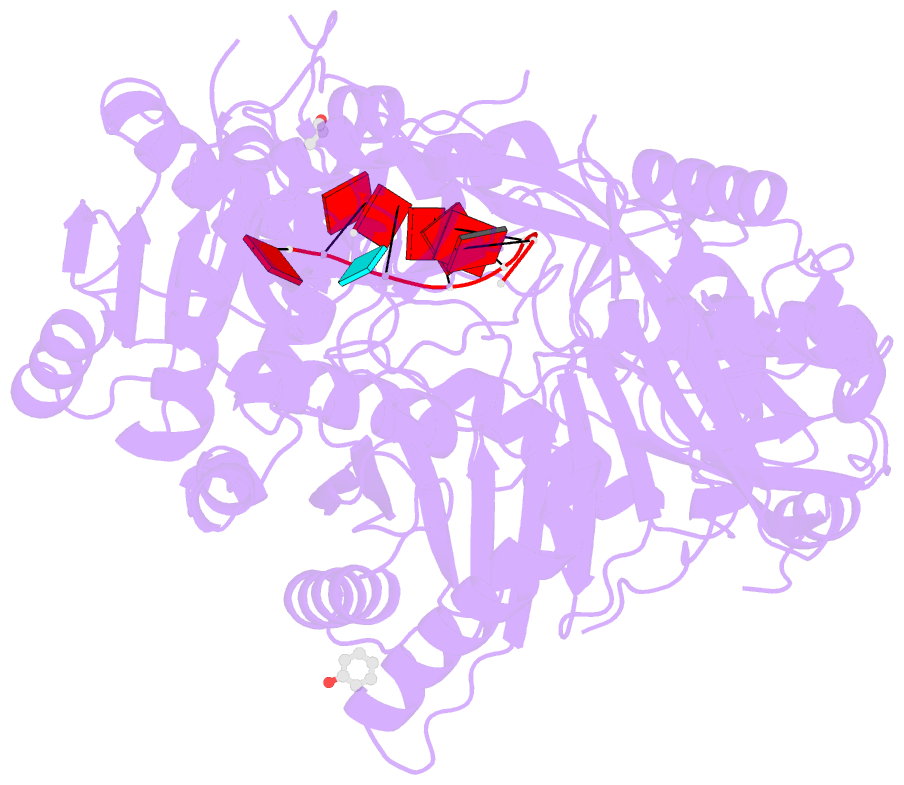
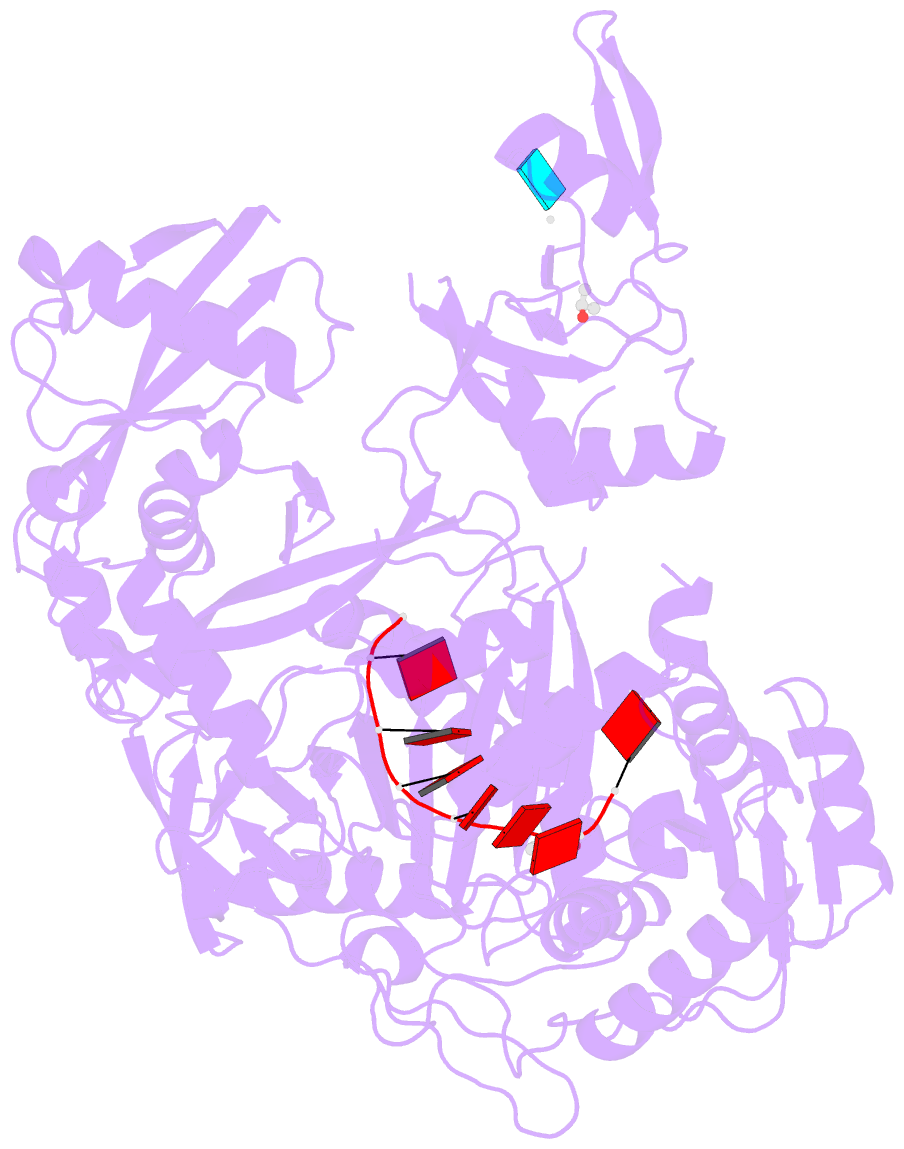
- Crystal structure of human argonaute2
- Reference
- Schirle NT, MacRae IJ (2012): "The crystal structure of human Argonaute2." Science, 336, 1037-1040. doi: 10.1126/science.1221551.
- Abstract
- Argonaute proteins form the functional core of the RNA-induced silencing complexes that mediate RNA silencing in eukaryotes. The 2.3 angstrom resolution crystal structure of human Argonaute2 (Ago2) reveals a bilobed molecule with a central cleft for binding guide and target RNAs. Nucleotides 2 to 6 of a heterogeneous mixture of guide RNAs are positioned in an A-form conformation for base pairing with target messenger RNAs. Between nucleotides 6 and 7, there is a kink that may function in microRNA target recognition or release of sliced RNA products. Tandem tryptophan-binding pockets in the PIWI domain define a likely interaction surface for recruitment of glycine-tryptophan-182 (GW182) or other tryptophan-rich cofactors. These results will enable structure-based approaches for harnessing the untapped therapeutic potential of RNA silencing in humans.