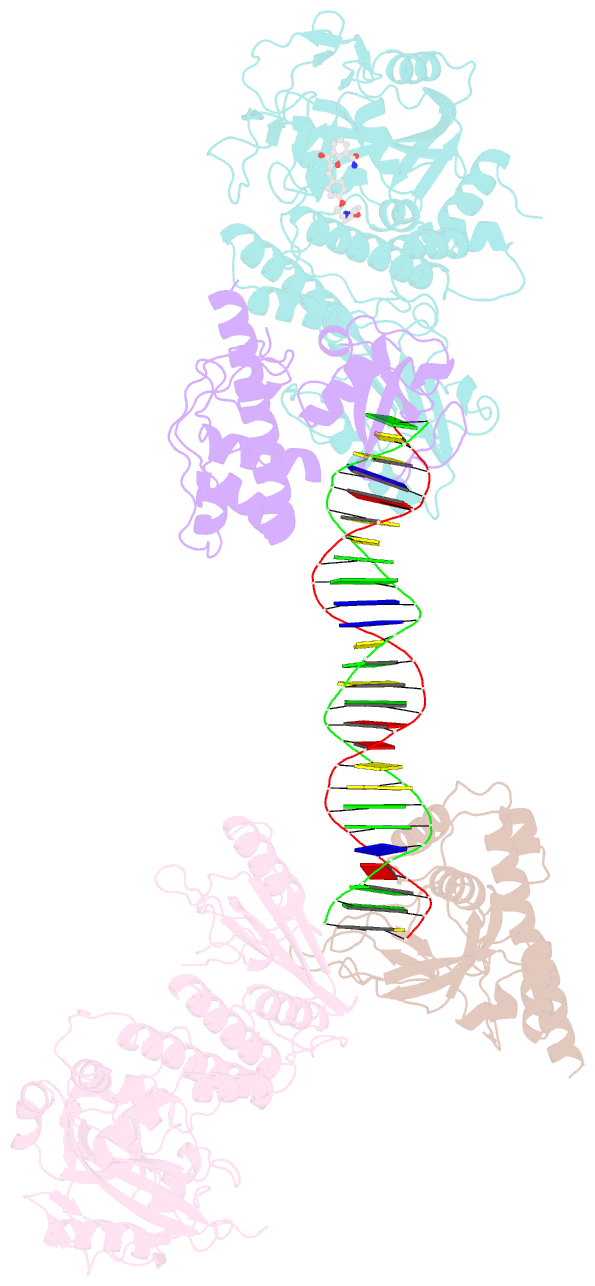
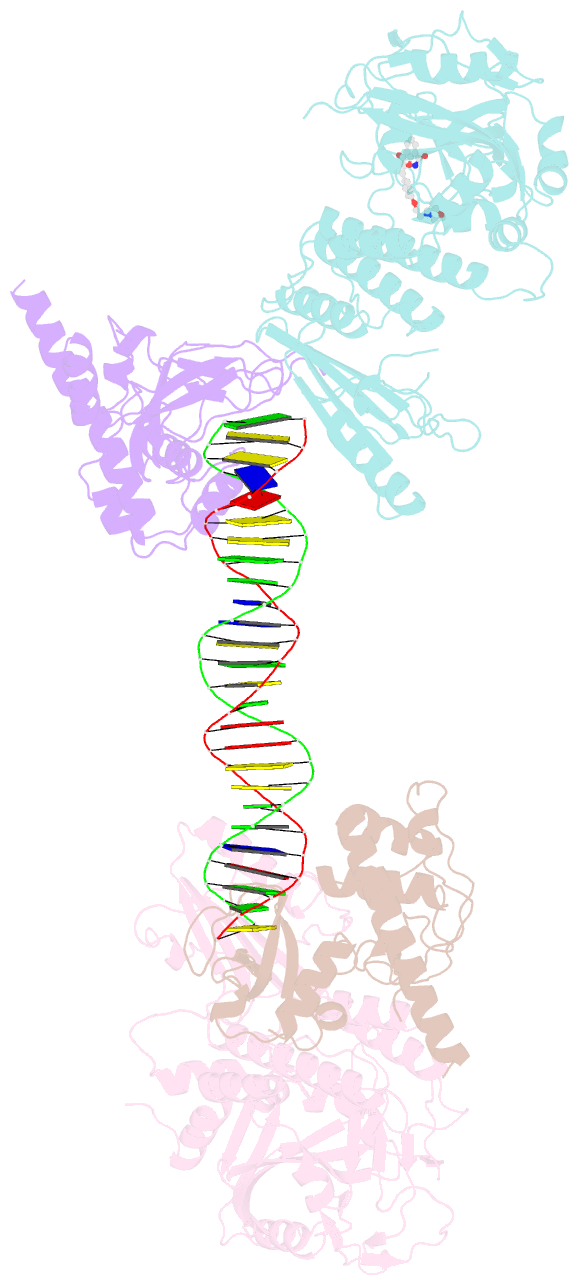
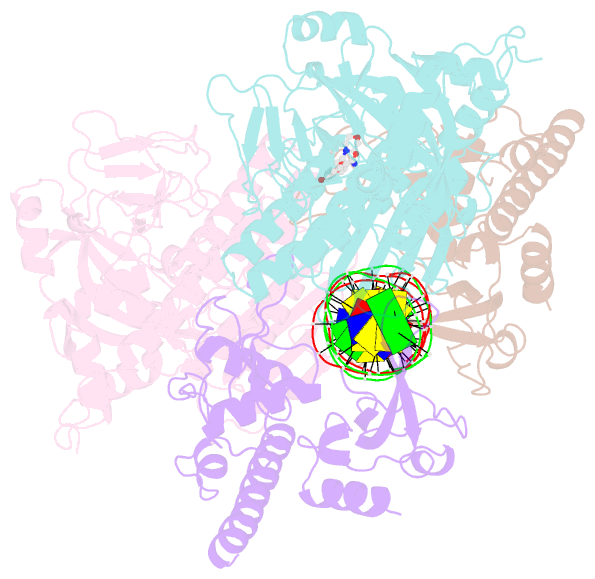
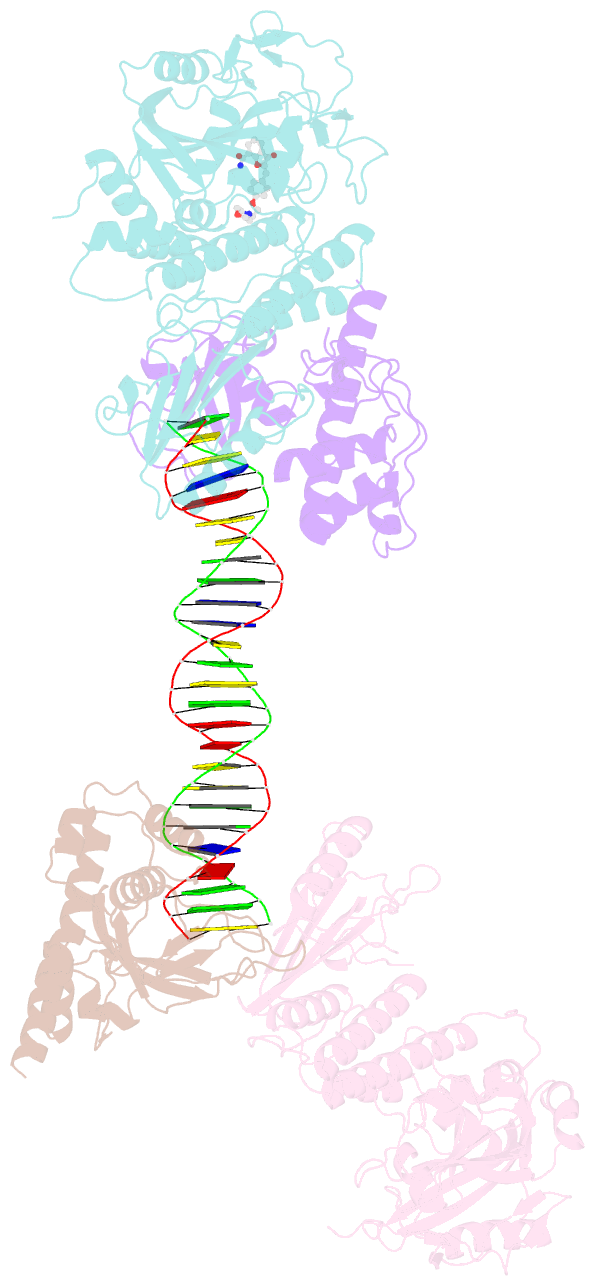
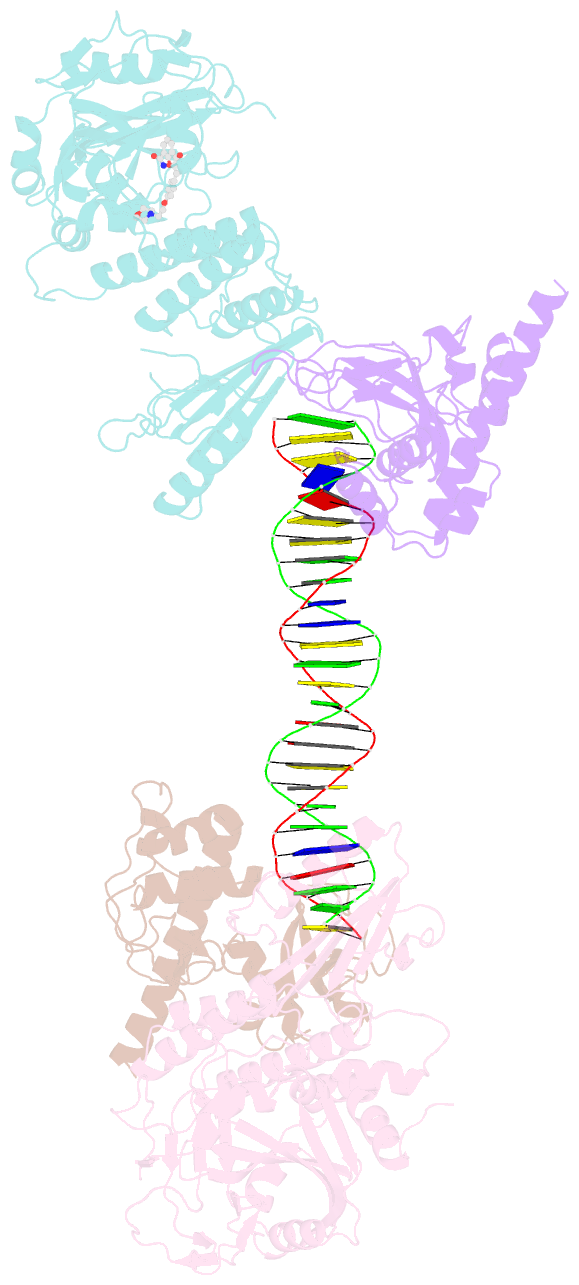
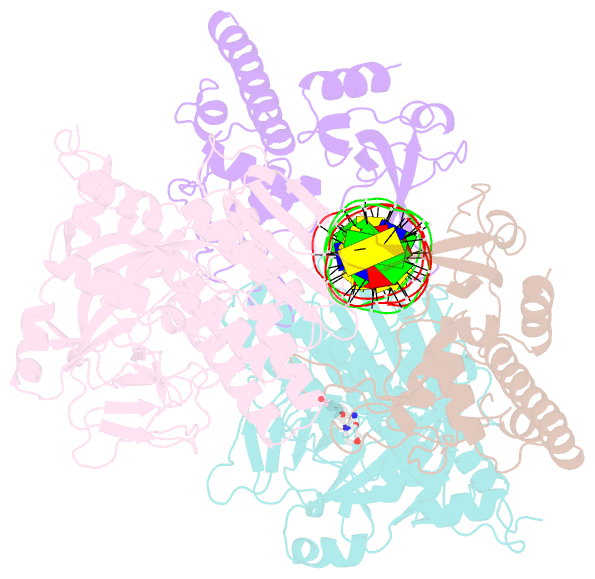
Summary information and primary citation
- PDB-id
- 4oqb; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA-transferase inhibitor
- Method
- X-ray (3.362 Å)
- Summary
- Structure of human parp-1 bound to a DNA double strand break in complex with (2z)-2-{4-[2-(morpholin-4-yl)ethoxy]benzylidene}-3-oxo-2,3-dihydro-1-benzofuran-7-carboxamide
- Reference
- Patel MR, Bhatt A, Steffen JD, Chergui A, Murai J, Pommier Y, Pascal JM, Trombetta LD, Fronczek FR, Talele TT (2014): "Discovery and Structure-Activity Relationship of Novel 2,3-Dihydrobenzofuran-7-carboxamide and 2,3-Dihydrobenzofuran-3(2H)-one-7-carboxamide Derivatives as Poly(ADP-ribose)polymerase-1 Inhibitors." J.Med.Chem., 57, 5579-5601. doi: 10.1021/jm5002502.
- Abstract
- Novel substituted 2,3-dihydrobenzofuran-7-carboxamide (DHBF-7-carboxamide) and 2,3-dihydrobenzofuran-3(2H)-one-7-carboxamide (DHBF-3-one-7-carboxamide) derivatives were synthesized and evaluated as inhibitors of poly(ADP-ribose)polymerase-1 (PARP-1). A structure-based design strategy resulted in lead compound 3 (DHBF-7-carboxamide; IC50 = 9.45 μM). To facilitate synthetically feasible derivatives, an alternative core was designed, DHBF-3-one-7-carboxamide (36, IC50 = 16.2 μM). The electrophilic 2-position of this scaffold was accessible for extended modifications. Substituted benzylidene derivatives at the 2-position were found to be the most potent, with 3',4'-dihydroxybenzylidene 58 (IC50 = 0.531 μM) showing a 30-fold improvement in potency. Various heterocycles attached at the 4'-hydroxyl/4'-amino of the benzylidene moiety resulted in significant improvement in inhibition of PARP-1 activity (e.g., compounds 66-68, 70, 72, and 73; IC50 values from 0.718 to 0.079 μM). Compound 66 showed selective cytotoxicity in BRCA2-deficient DT40 cells. Crystal structures of three inhibitors (compounds (-)-13c, 59, and 65) bound to a multidomain PARP-1 structure were obtained, providing insights into further development of these inhibitors.