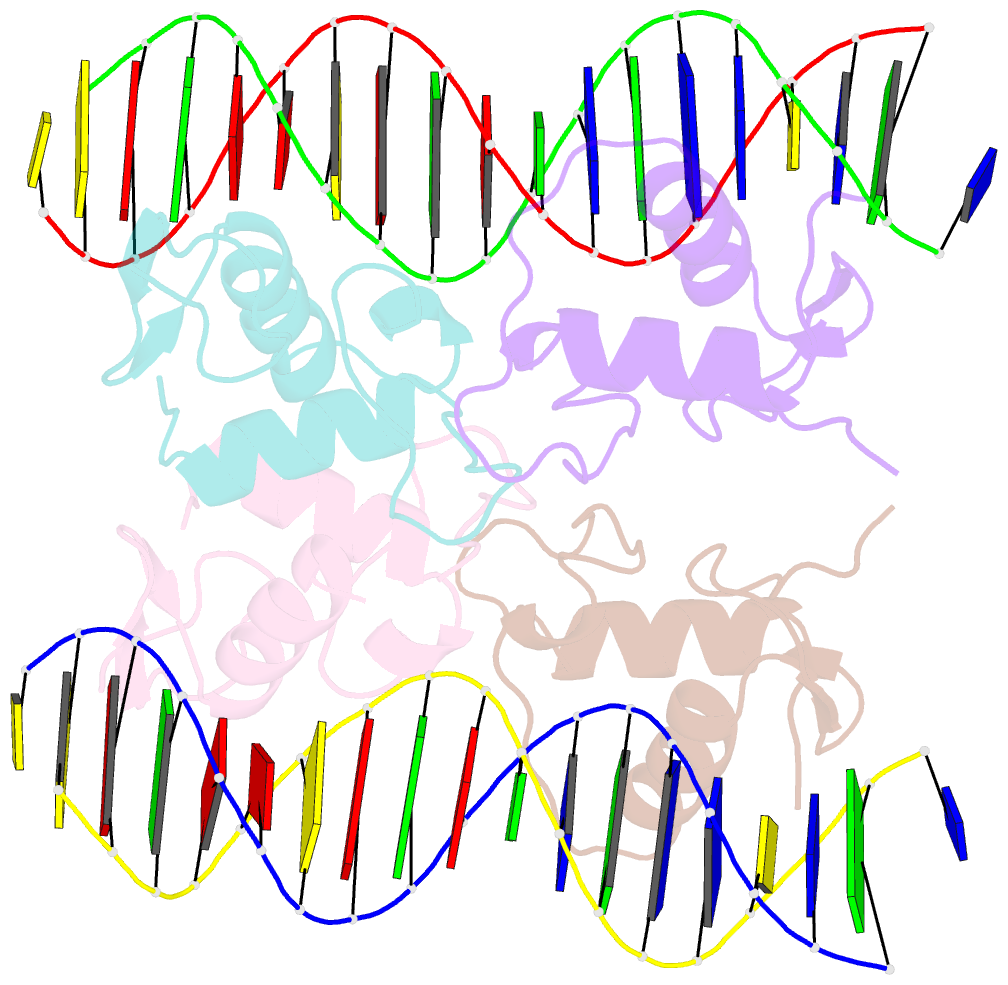
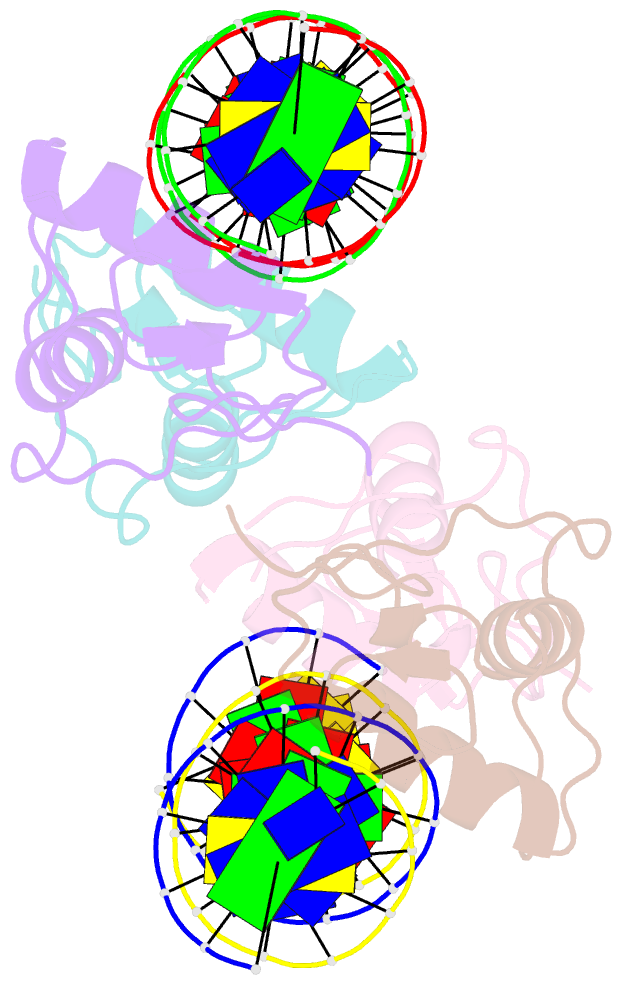
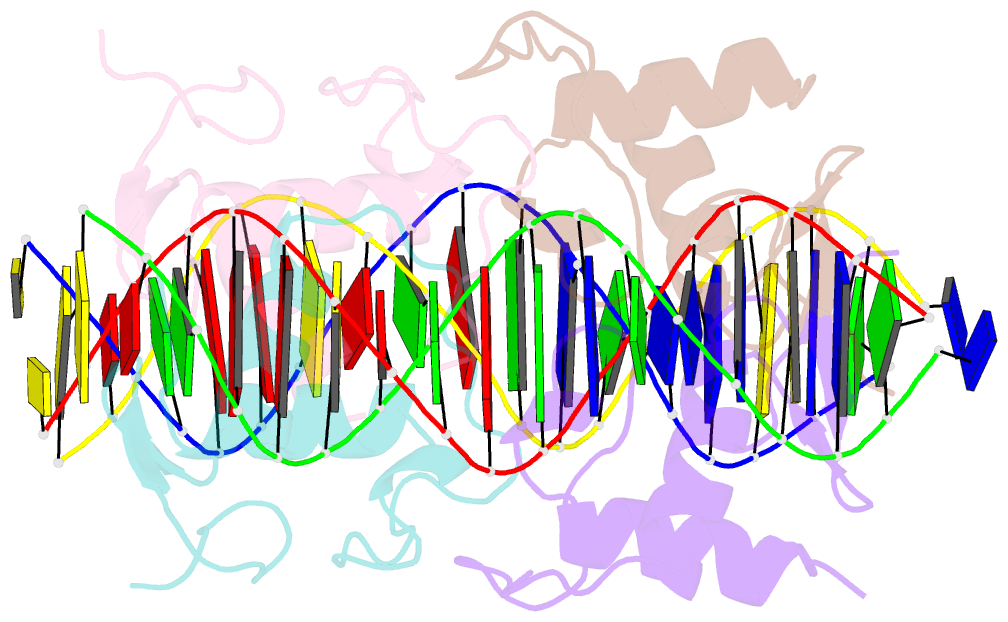
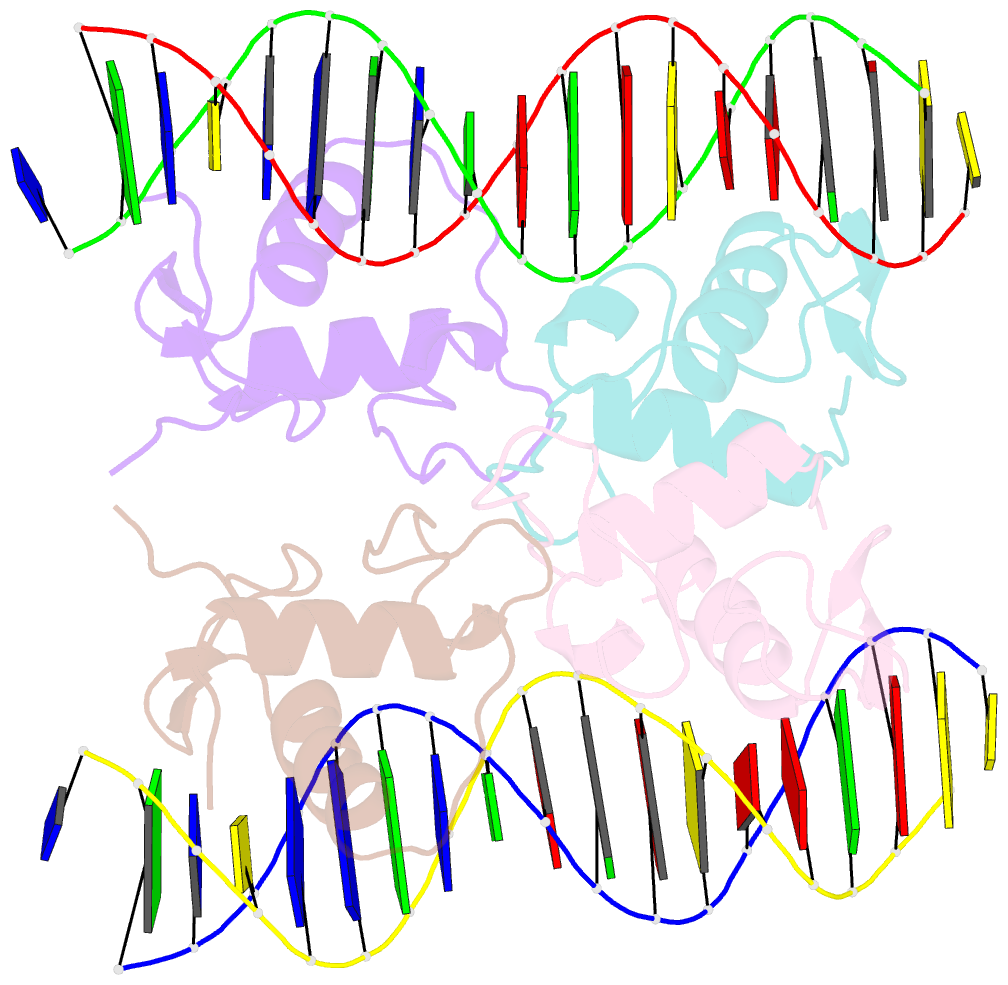
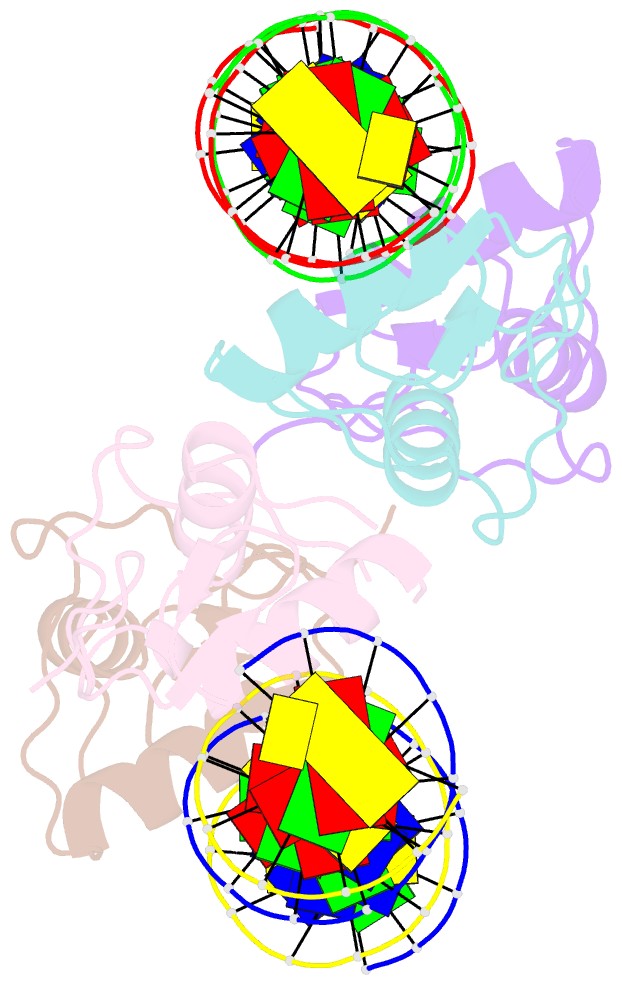
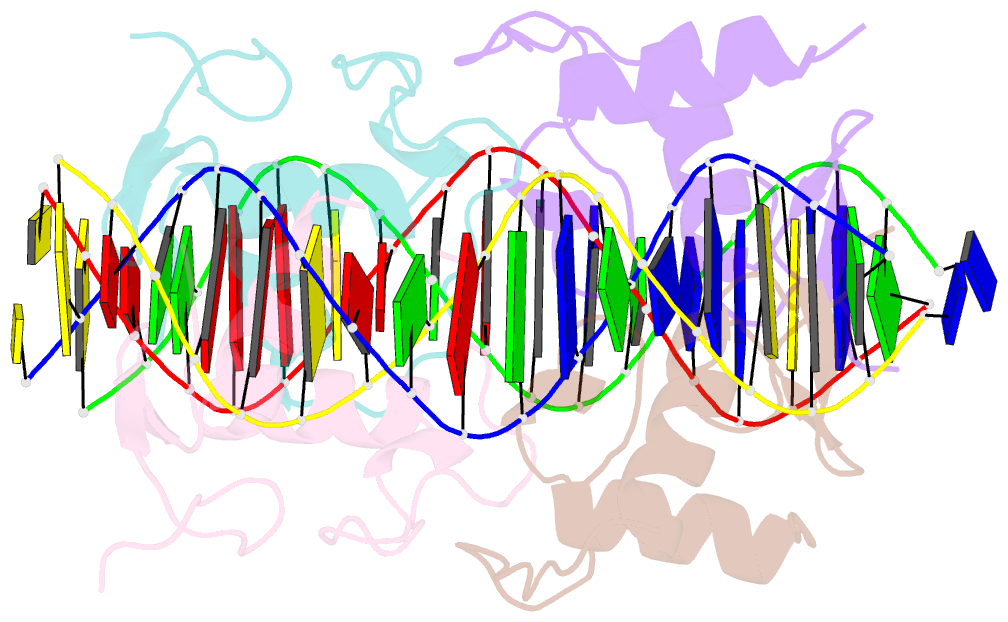
Summary information and primary citation
- PDB-id
- 4ov7; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.701 Å)
- Summary
- Ancestral steroid receptor 2 dbd helix mutant - sre DNA complex
- Reference
- McKeown AN, Bridgham JT, Anderson DW, Murphy MN, Ortlund EA, Thornton JW (2014): "Evolution of DNA specificity in a transcription factor family produced a new gene regulatory module." Cell(Cambridge,Mass.), 159, 58-68. doi: 10.1016/j.cell.2014.09.003.
- Abstract
- Complex gene regulatory networks require transcription factors (TFs) to bind distinct DNA sequences. To understand how novel TF specificity evolves, we combined phylogenetic, biochemical, and biophysical approaches to interrogate how DNA recognition diversified in the steroid hormone receptor (SR) family. After duplication of the ancestral SR, three mutations in one copy radically weakened binding to the ancestral estrogen response element (ERE) and improved binding to a new set of DNA sequences (steroid response elements, SREs). They did so by establishing unfavorable interactions with ERE and abolishing unfavorable interactions with SRE; also required were numerous permissive substitutions, which nonspecifically improved cooperativity and affinity of DNA binding. Our findings indicate that negative determinants of binding play key roles in TFs' DNA selectivity and-with our prior work on the evolution of SR ligand specificity during the same interval-show how a specific new gene regulatory module evolved without interfering with the integrity of the ancestral module.