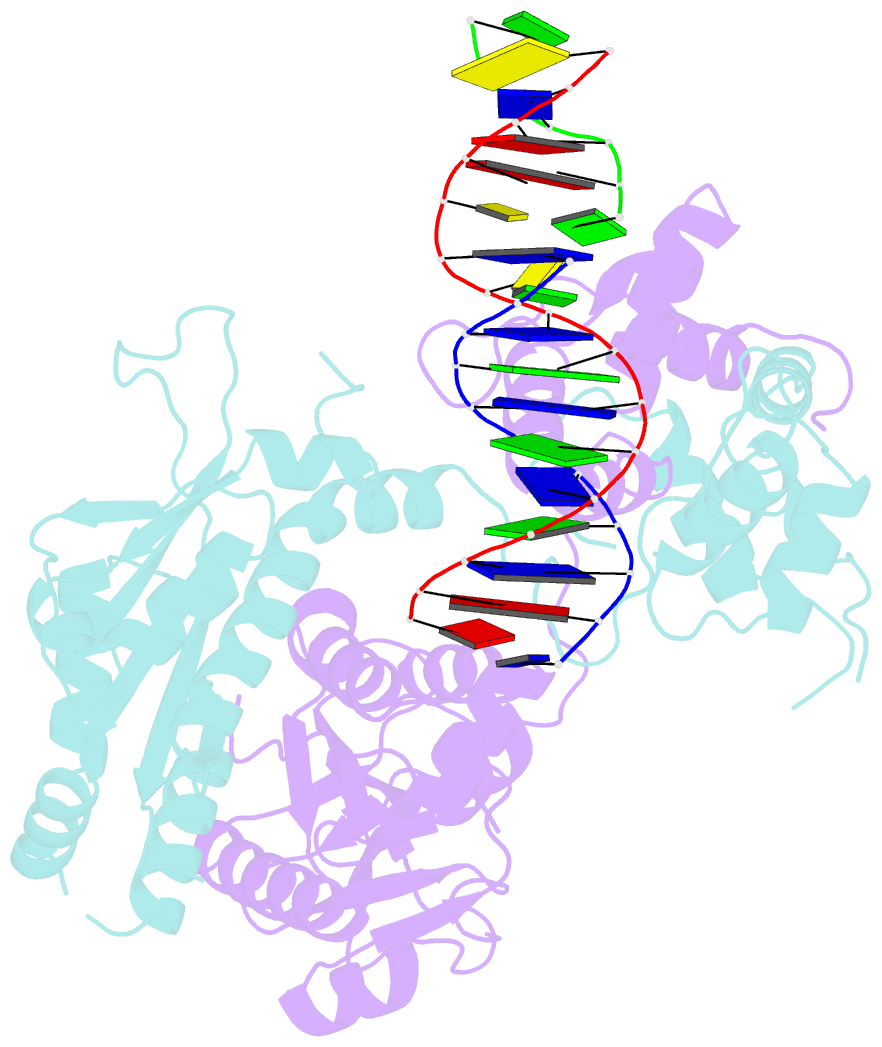
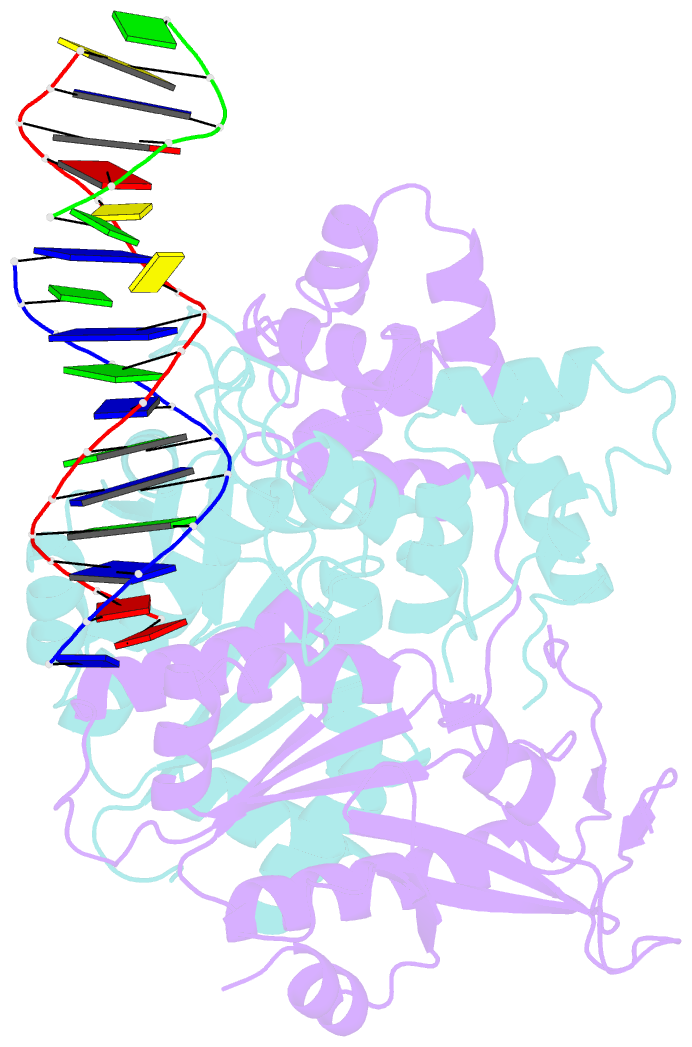
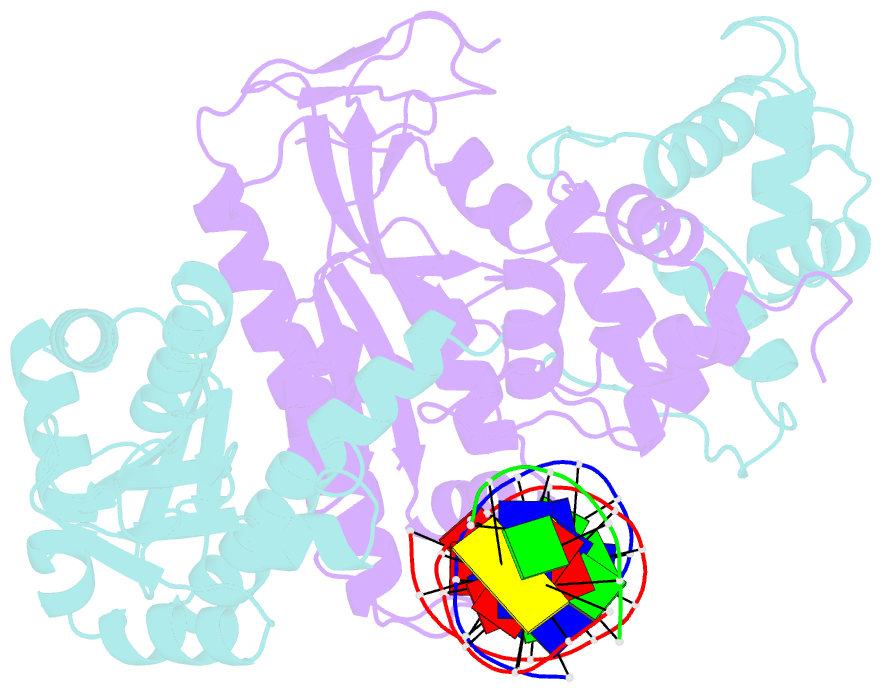
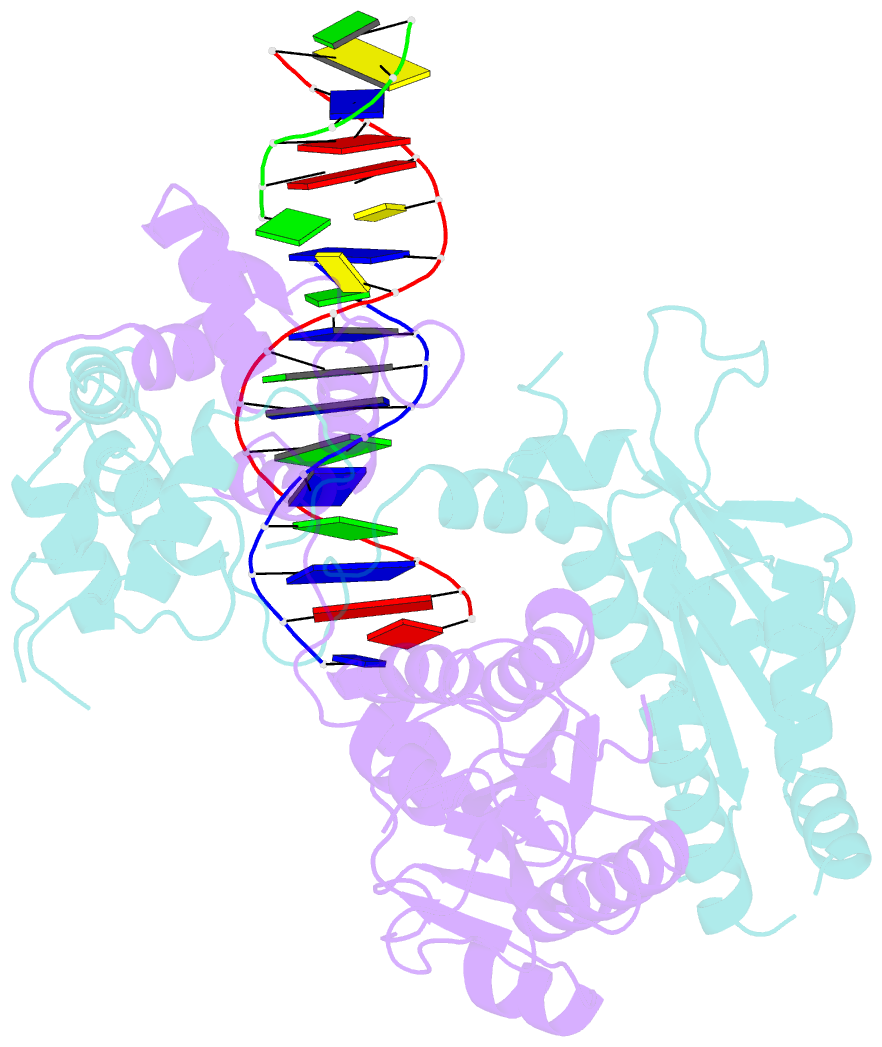
Summary information and primary citation
- PDB-id
- 4p0p; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.8 Å)
- Summary
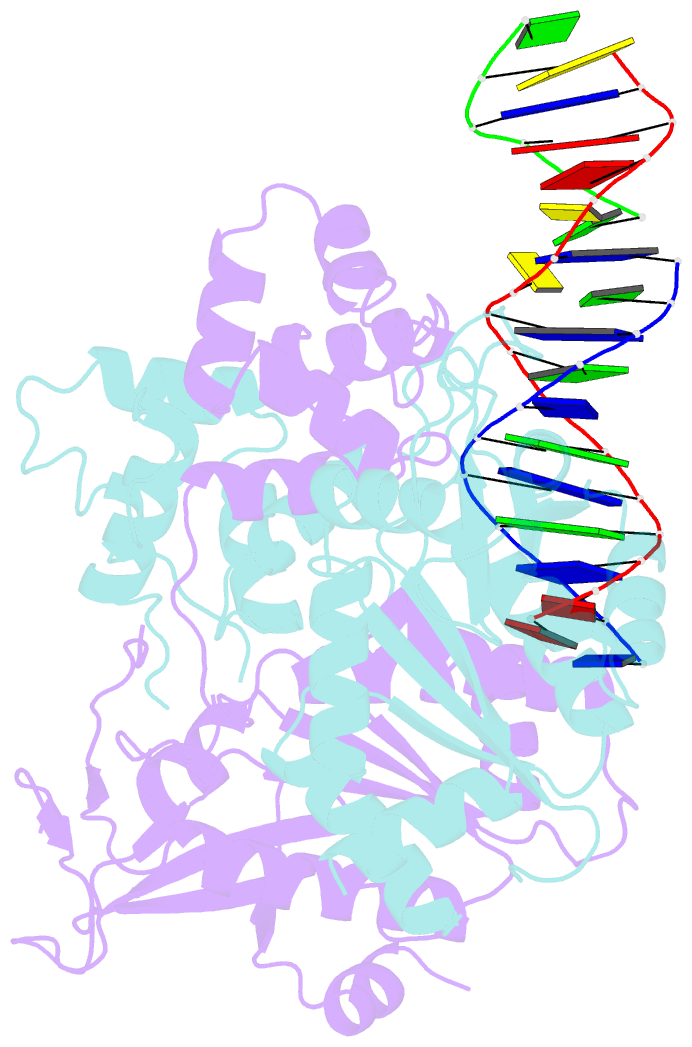
- Crystal structure of human mus81-eme1 in complex with 5'-flap DNA, and mg2+
- Reference
- Gwon GH, Jo A, Baek K, Jin KS, Fu Y, Lee JB, Kim Y, Cho Y (2014): "Crystal structures of the structure-selective nuclease Mus81-Eme1 bound to flap DNA substrates." Embo J., 33, 1061-1072. doi: 10.1002/embj.201487820.
- Abstract
- The Mus81-Eme1 complex is a structure-selective endonuclease with a critical role in the resolution of recombination intermediates during DNA repair after interstrand cross-links, replication fork collapse, or double-strand breaks. To explain the molecular basis of 3' flap substrate recognition and cleavage mechanism by Mus81-Eme1, we determined crystal structures of human Mus81-Eme1 bound to various flap DNA substrates. Mus81-Eme1 undergoes gross substrate-induced conformational changes that reveal two key features: (i) a hydrophobic wedge of Mus81 that separates pre- and post-nick duplex DNA and (ii) a "5' end binding pocket" that hosts the 5' nicked end of post-nick DNA. These features are crucial for comprehensive protein-DNA interaction, sharp bending of the 3' flap DNA substrate, and incision strand placement at the active site. While Mus81-Eme1 unexpectedly shares several common features with members of the 5' flap nuclease family, the combined structural, biochemical, and biophysical analyses explain why Mus81-Eme1 preferentially cleaves 3' flap DNA substrates with 5' nicked ends.