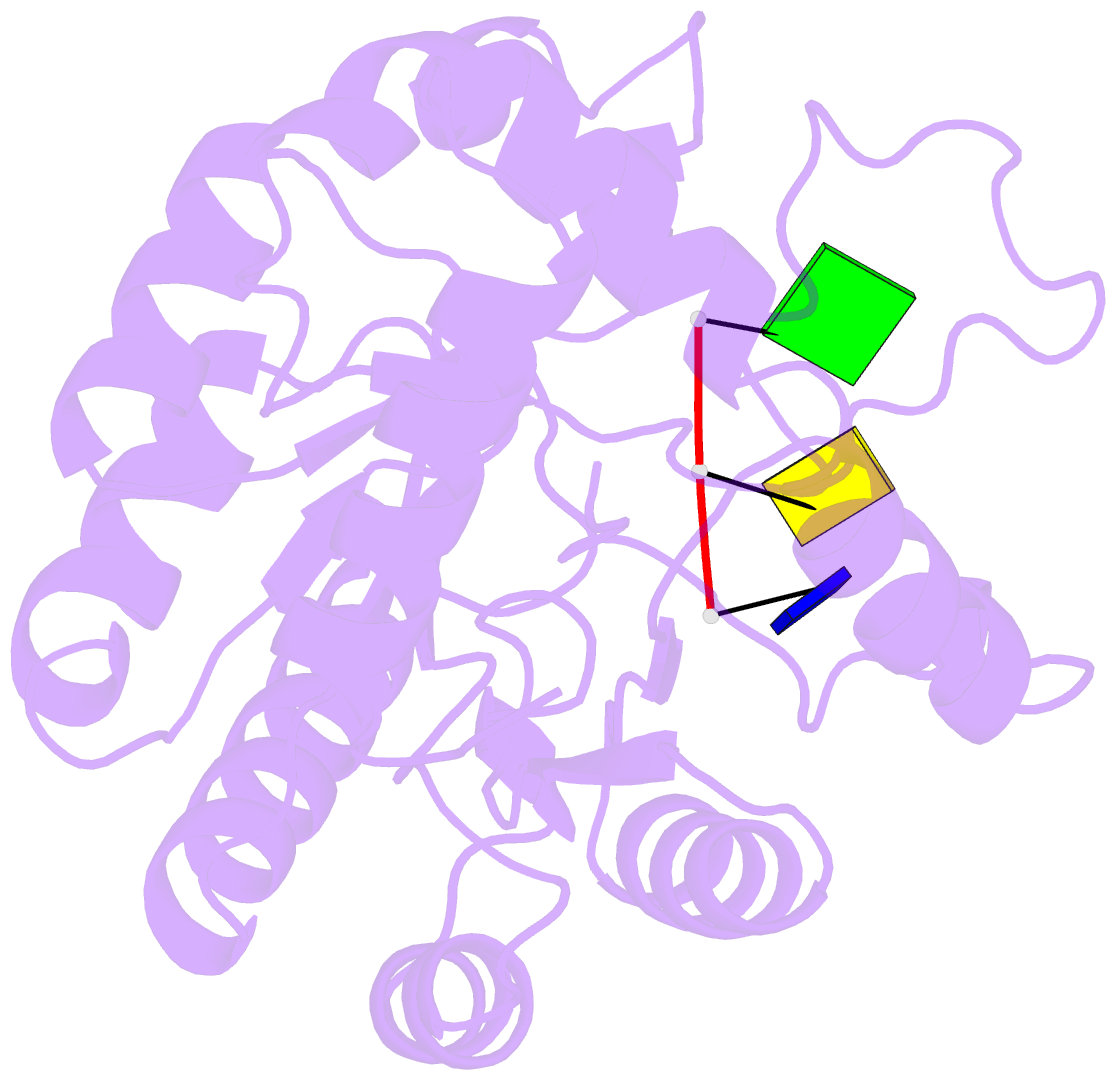
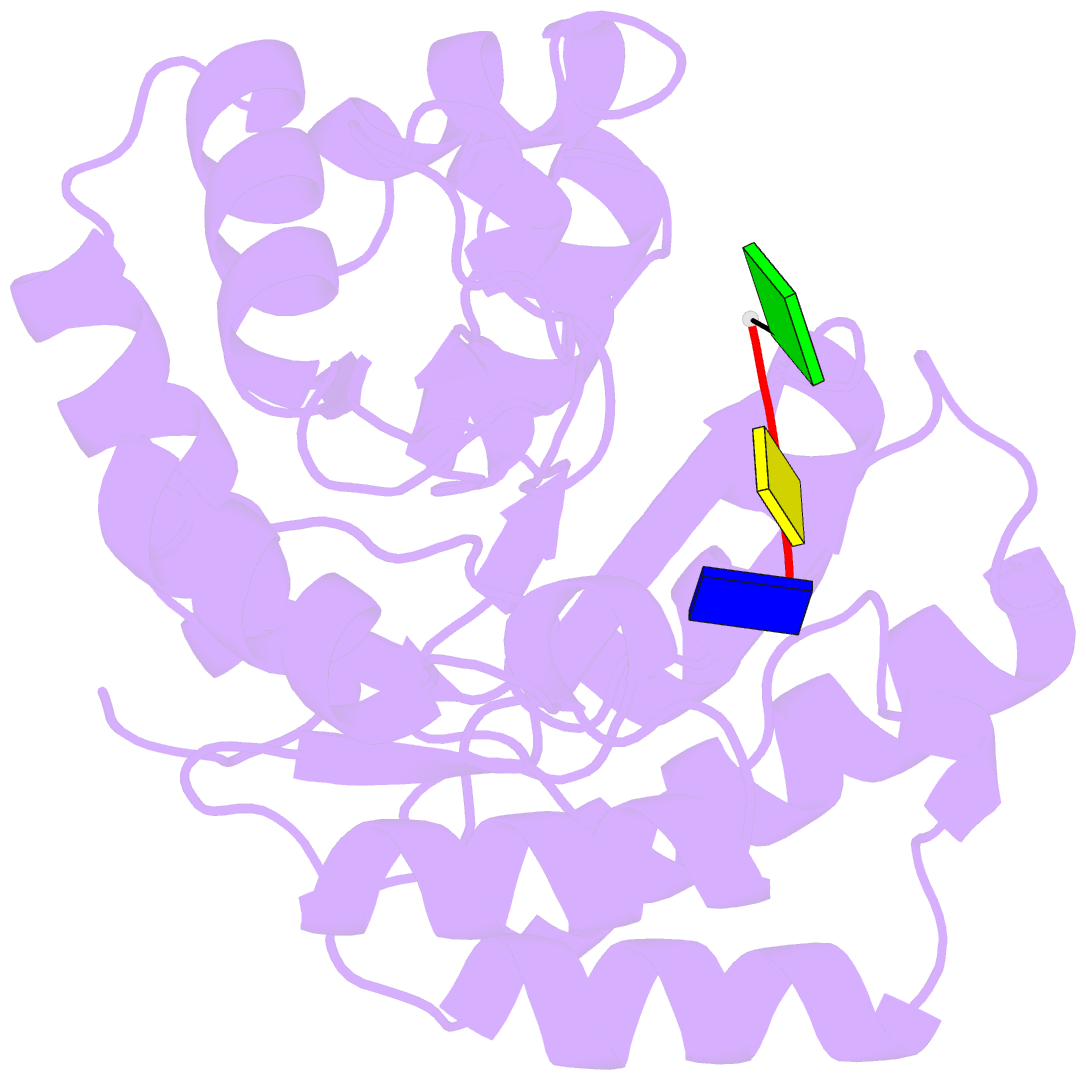
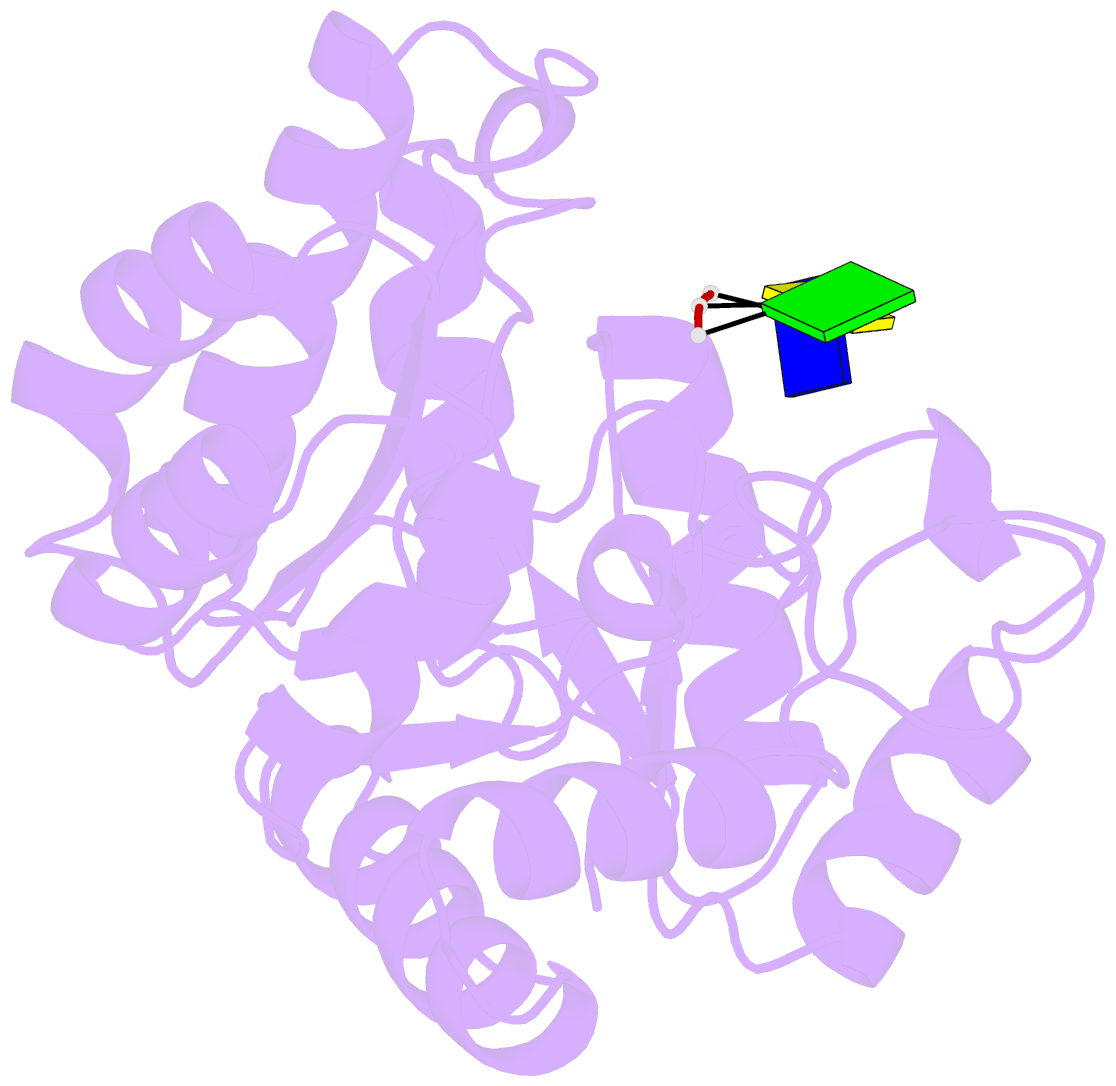
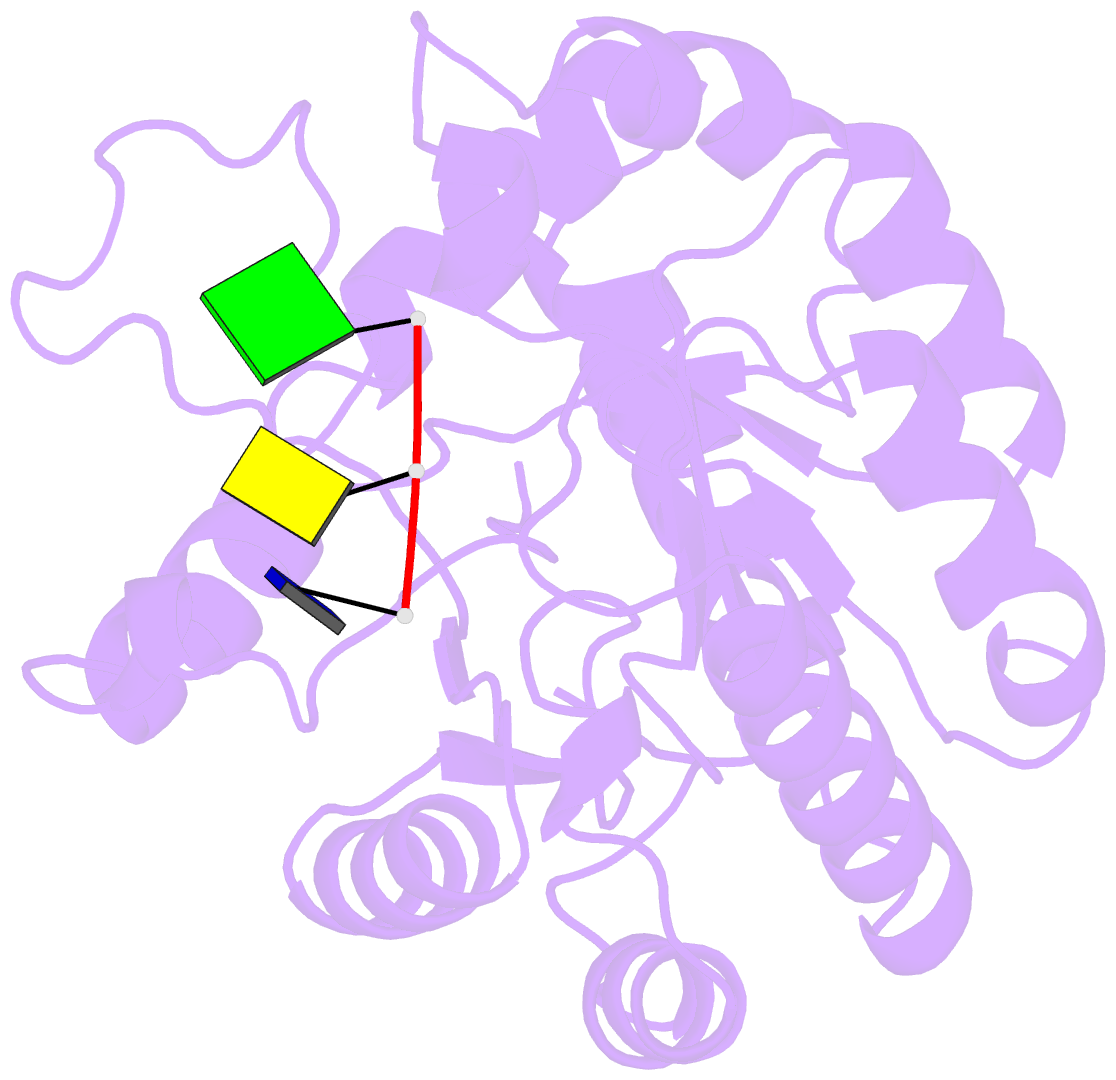
Summary information and primary citation
- PDB-id
- 4pe8; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.894 Å)
- Summary
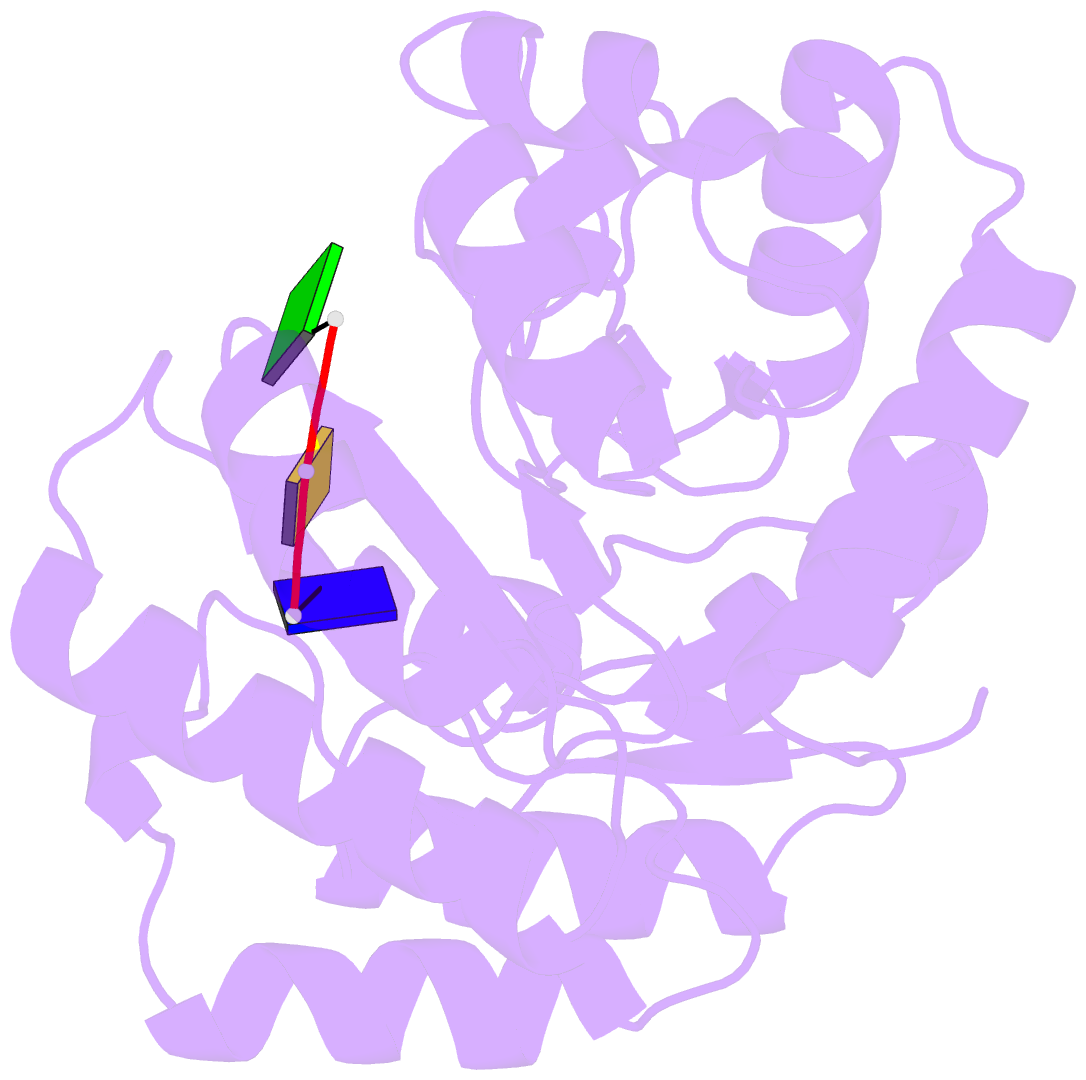
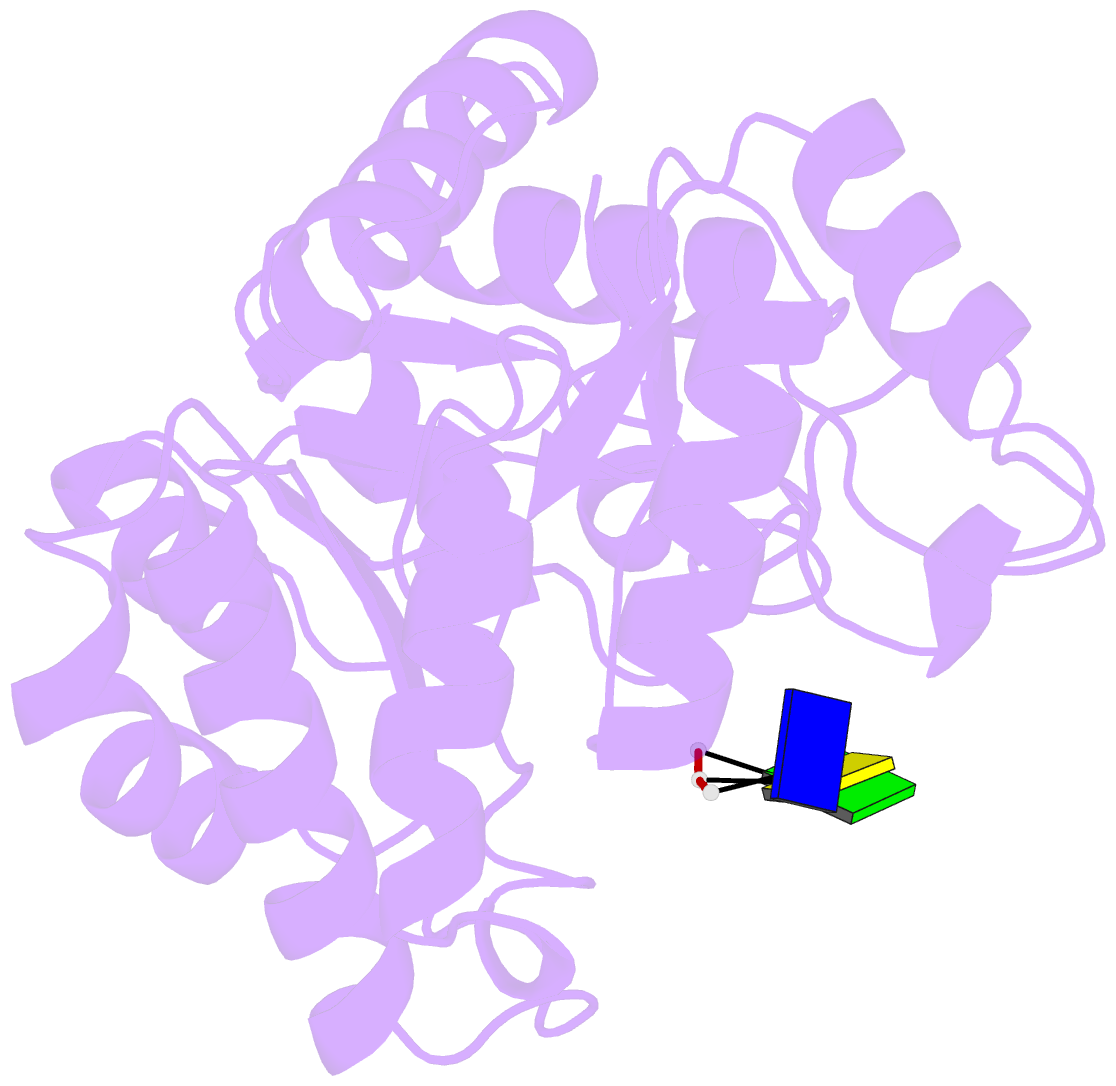
- Crystal structure of tatd in complex with trinucleotide DNA
- Reference
- Chen YC, Li CL, Hsiao YY, Duh Y, Yuan HS (2014): "Structure and function of TatD exonuclease in DNA repair." Nucleic Acids Res., 42, 10776-10785. doi: 10.1093/nar/gku732.
- Abstract
- TatD is an evolutionarily conserved protein with thousands of homologues in all kingdoms of life. It has been suggested that TatD participates in DNA fragmentation during apoptosis in eukaryotic cells. However, the cellular functions and biochemical properties of TatD in bacterial and non-apoptotic eukaryotic cells remain elusive. Here we show that Escherichia coli TatD is a Mg(2+)-dependent 3'-5' exonuclease that prefers to digest single-stranded DNA and RNA. TatD-knockout cells are less resistant to the DNA damaging agent hydrogen peroxide, and TatD can remove damaged deaminated nucleotides from a DNA chain, suggesting that it may play a role in the H2O2-induced DNA repair. The crystal structure of the apo-form TatD and TatD bound to a single-stranded three-nucleotide DNA was determined by X-ray diffraction methods at a resolution of 2.0 and 2.9 Å, respectively. TatD has a TIM-barrel fold and the single-stranded DNA is bound at the loop region on the top of the barrel. Mutational studies further identify important conserved metal ion-binding and catalytic residues in the TatD active site for DNA hydrolysis. We thus conclude that TatD is a new class of TIM-barrel 3'-5' exonuclease that not only degrades chromosomal DNA during apoptosis but also processes single-stranded DNA during DNA repair.