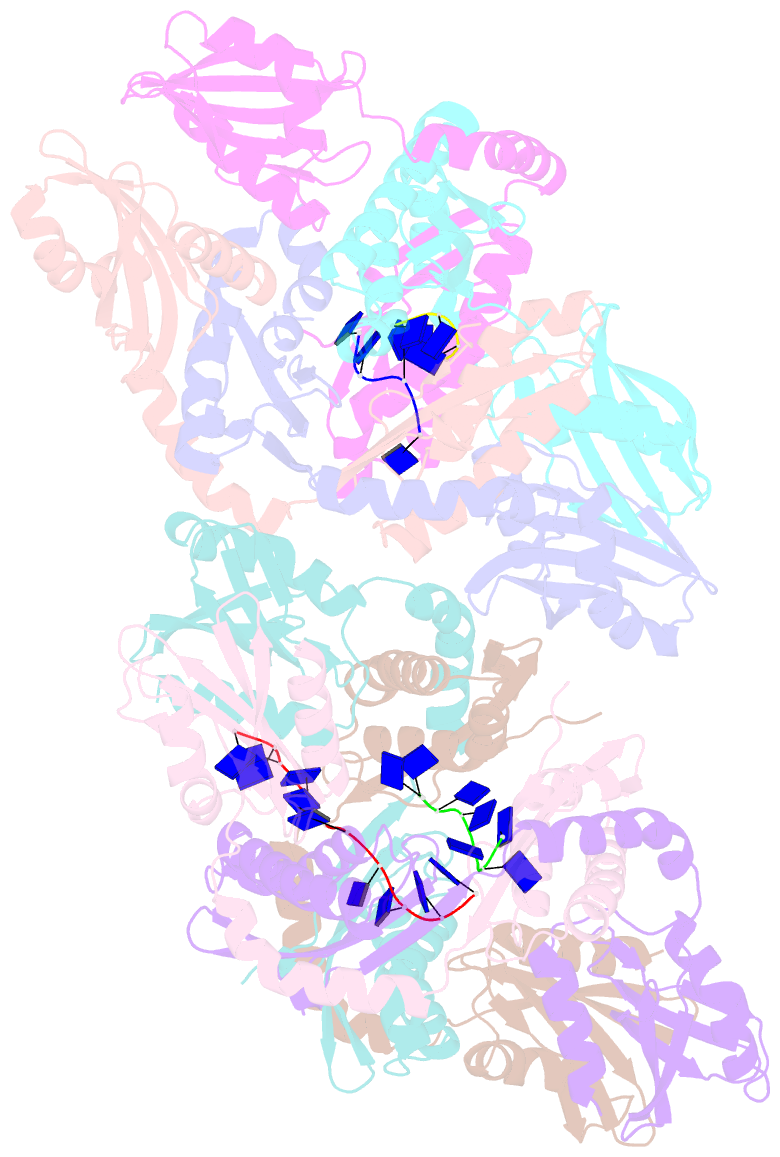
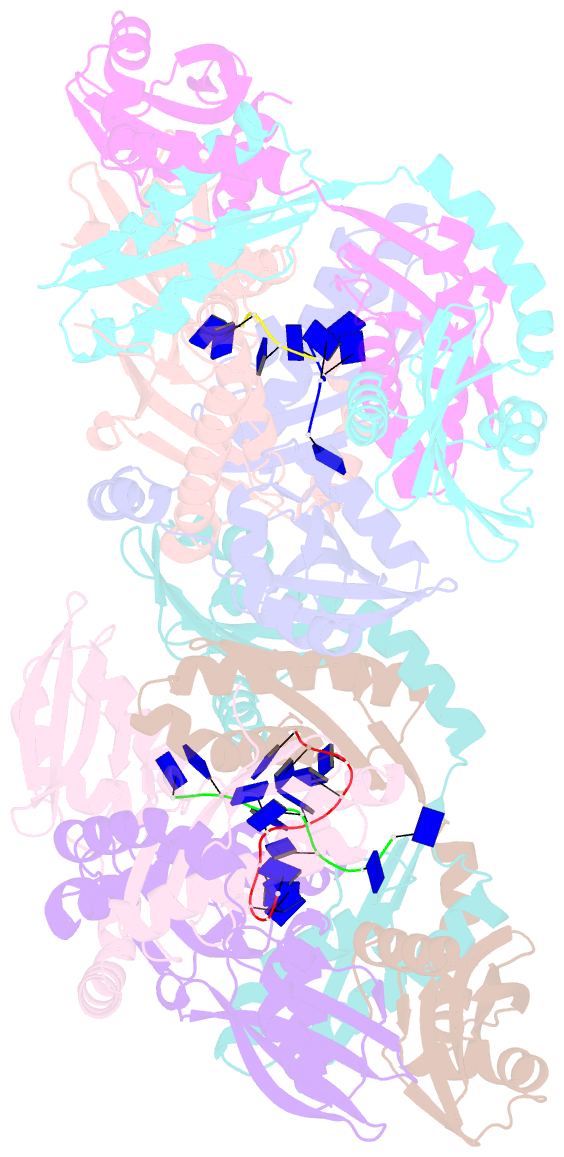
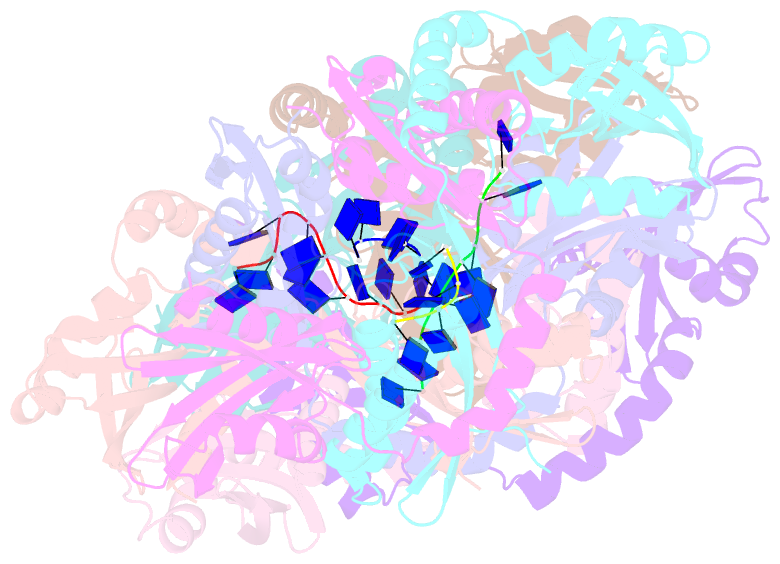
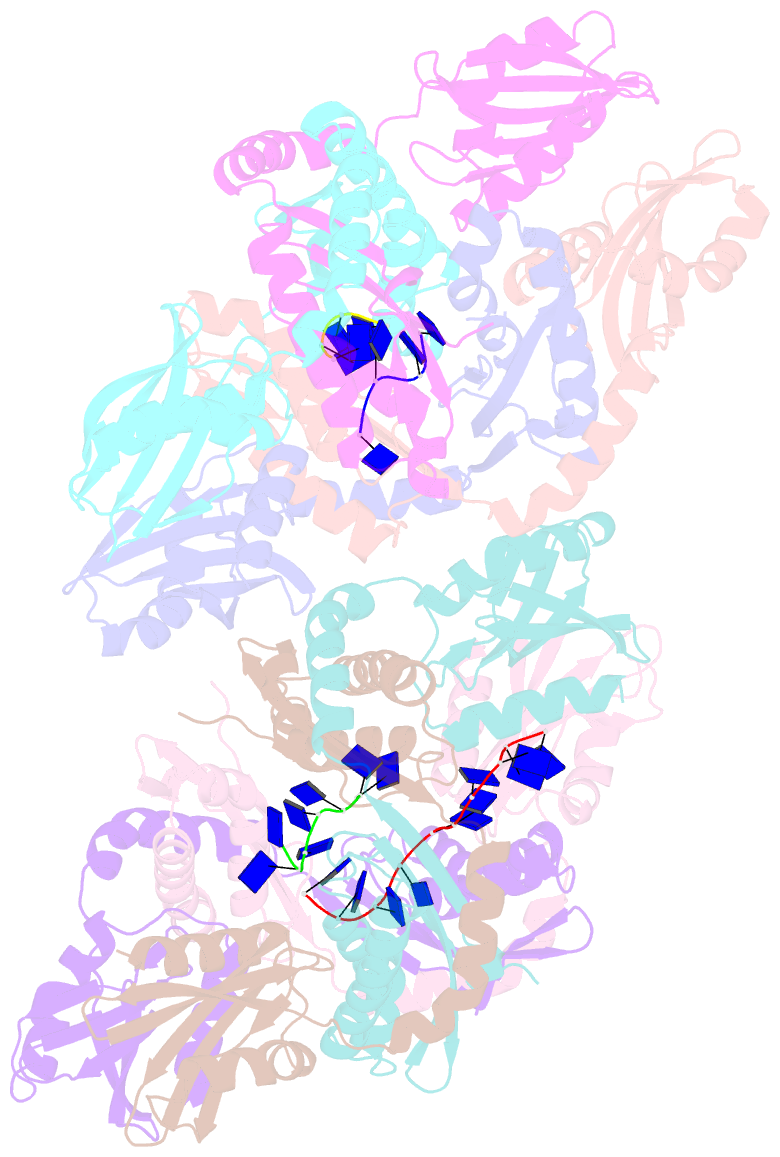
Summary information and primary citation
- PDB-id
- 4pso; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- X-ray (2.9 Å)
- Summary
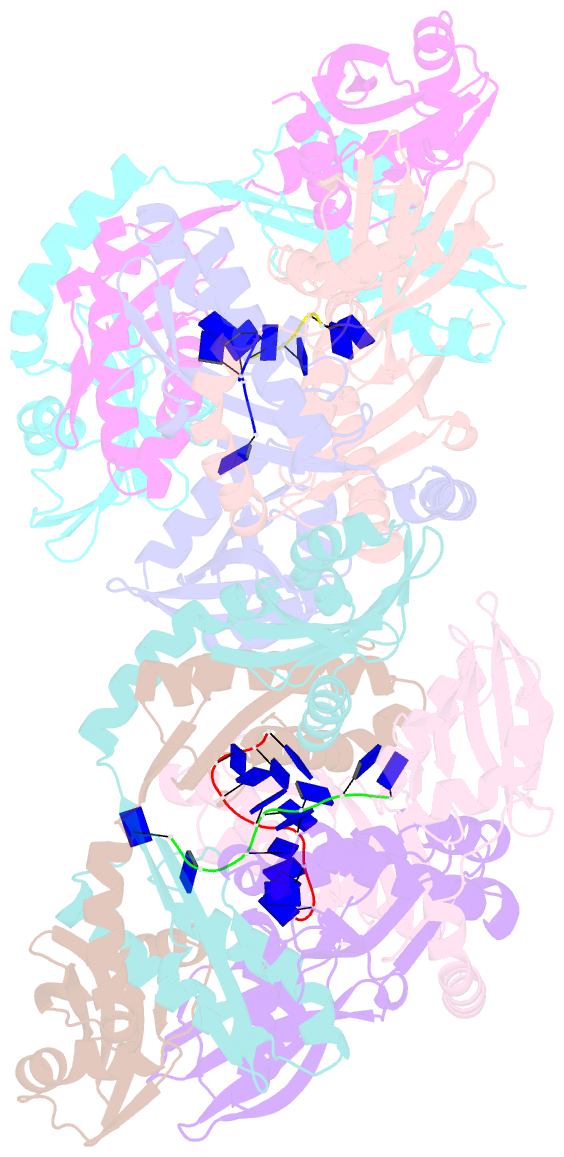
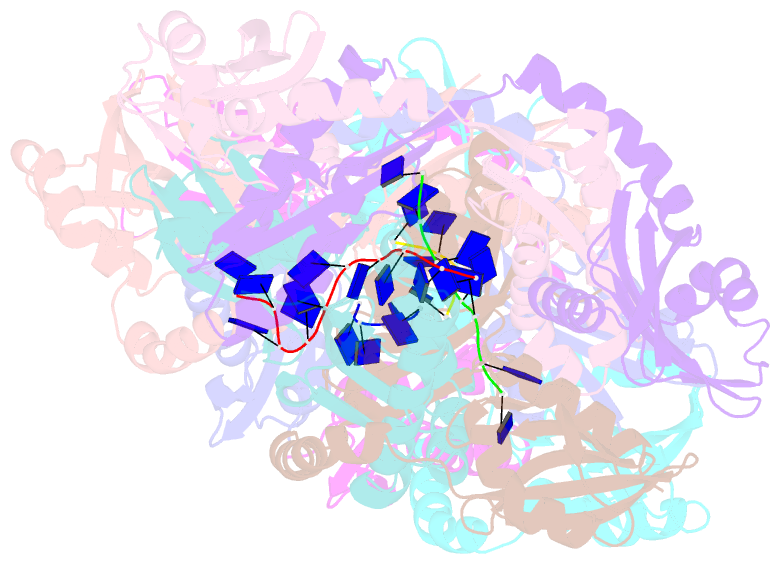
- Crystal structure of apethermo-dbp-rp2 bound to ssDNA dt10
- Reference
- Ghalei H, Moeller Hv, Eppers D, Sohmen D, Wilson DN, Loll B, Wahl MC (2014): "Entrapment of DNA in an intersubunit tunnel system of a single-stranded DNA-binding protein." Nucleic Acids Res., 42, 6698-6708. doi: 10.1093/nar/gku259.
- Abstract
- Instead of a classical single-stranded deoxyribonuleic acid (DNA)-binding protein (SSB), some hyperthermophilic crenarchaea harbor a non-canonical SSB termed ThermoDBP. Two related but poorly characterized groups of proteins, which share the ThermoDBP N-terminal DNA-binding domain, have a broader phylogenetic distribution and co-exist with ThermoDBPs and/or other SSBs. We have investigated the nucleic acid binding properties and crystal structures of representatives of these groups of ThermoDBP-related proteins (ThermoDBP-RPs) 1 and 2. ThermoDBP-RP 1 and 2 oligomerize by different mechanisms and only ThermoDBP-RP2 exhibits strong single-stranded DNA affinity in vitro. A crystal structure of ThermoDBP-RP2 in complex with DNA reveals how the NTD common to ThermoDBPs and ThermoDBP-RPs can contact the nucleic acid in a manner that allows a symmetric homotetrameric protein complex to bind single-stranded DNA molecules asymmetrically. While single-stranded DNA wraps around the surface or binds along channels of previously investigated SSBs, it traverses an internal, intersubunit tunnel system of a ThermoDBP-RP2 tetramer. Our results indicate that some archaea have acquired special SSBs for genome maintenance in particularly challenging environments.