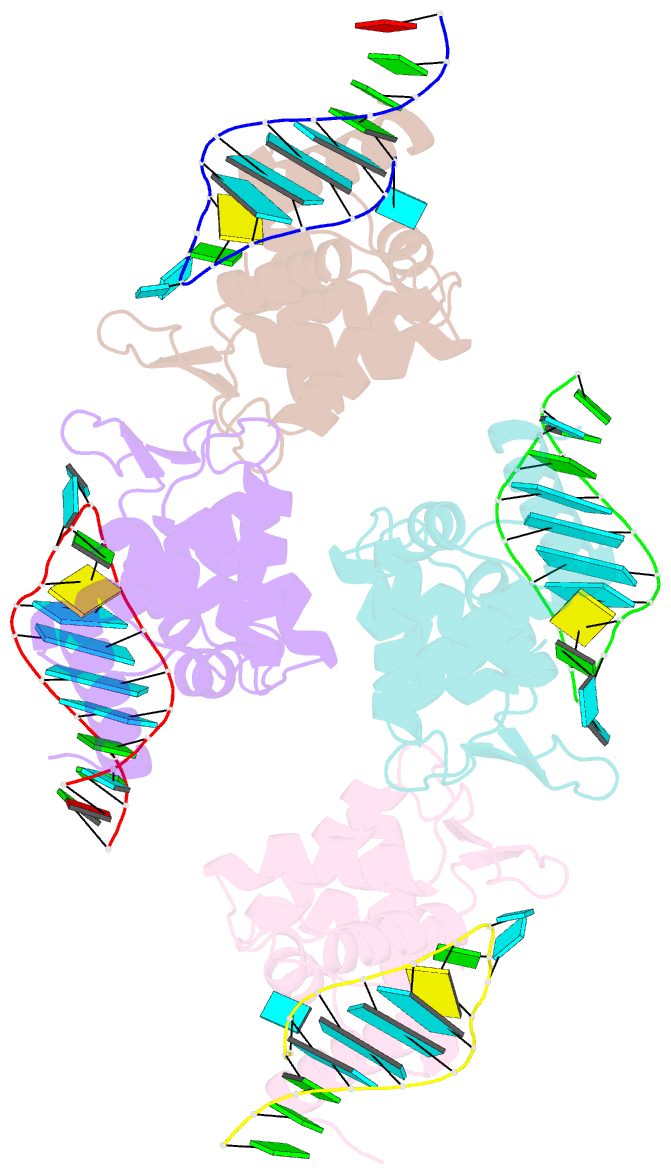
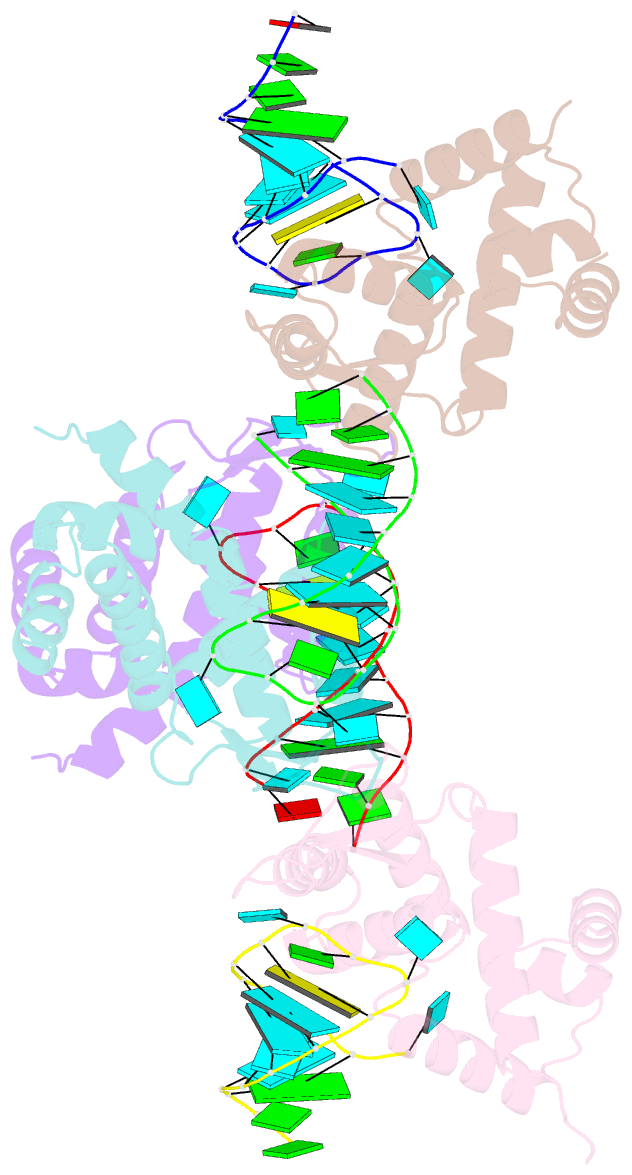
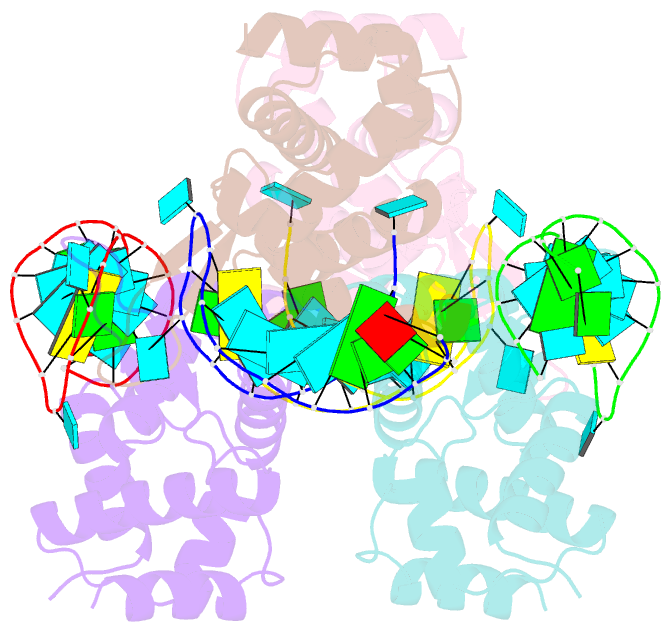
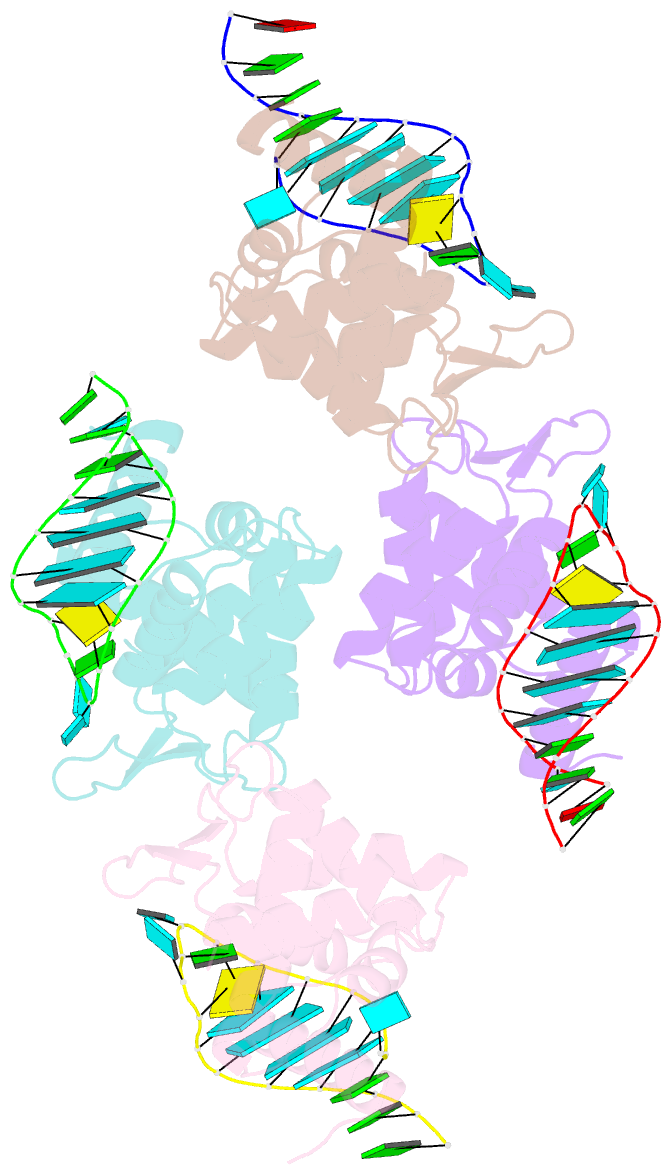
Summary information and primary citation
- PDB-id
- 4qi2; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (3.0 Å)
- Summary
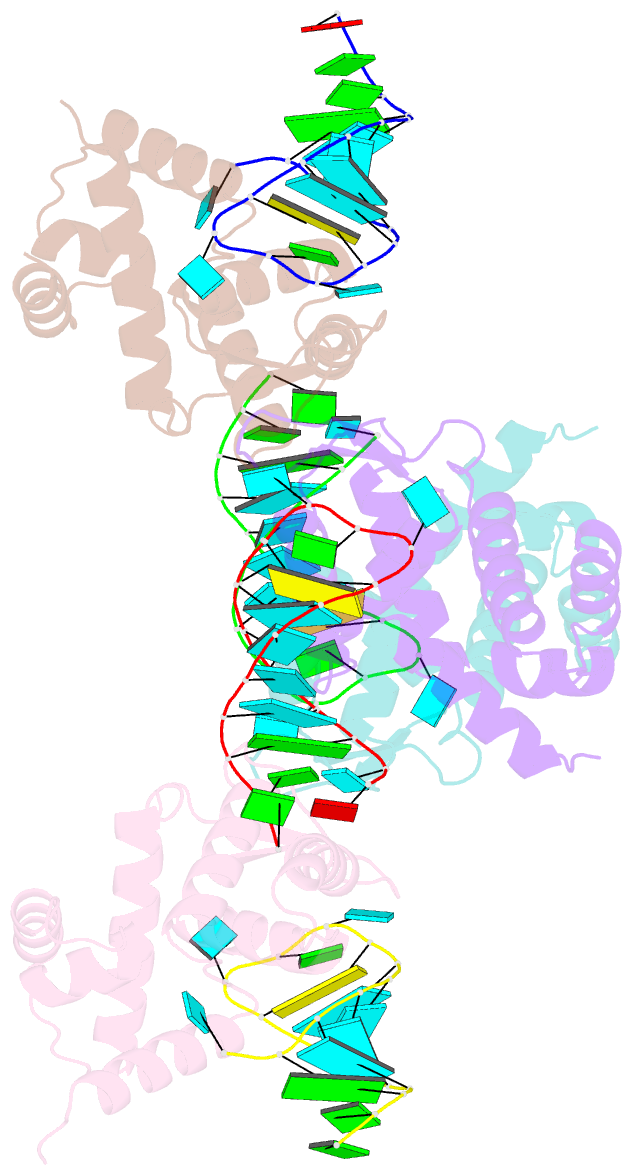
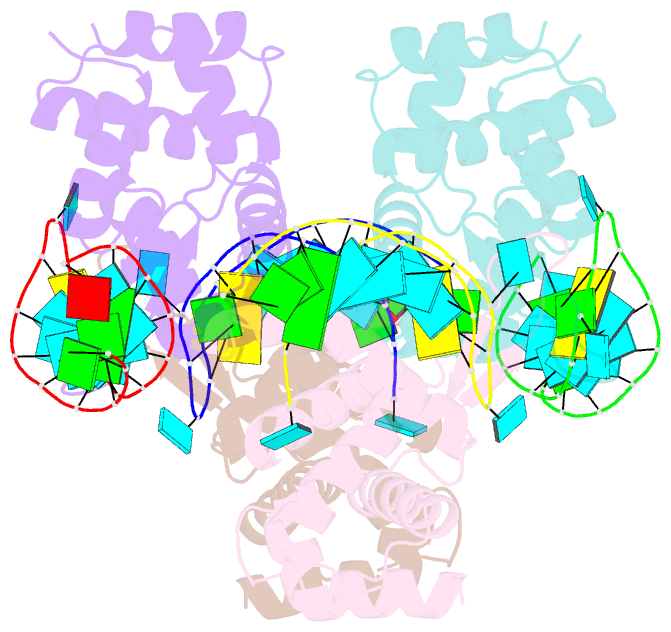
- X-ray structure of the roq domain from murine roquin-1 in complex with a 23-mer tnf-cde RNA
- Reference
- Schlundt A, Heinz GA, Janowski R, Geerlof A, Stehle R, Heissmeyer V, Niessing D, Sattler M (2014): "Structural basis for RNA recognition in roquin-mediated post-transcriptional gene regulation." Nat.Struct.Mol.Biol., 21, 671-678. doi: 10.1038/nsmb.2855.
- Abstract
- Roquin function in T cells is essential for the prevention of autoimmune disease. Roquin interacts with the 3' untranslated regions (UTRs) of co-stimulatory receptors and controls T-cell activation and differentiation. Here we show that the N-terminal ROQ domain from mouse roquin adopts an extended winged-helix (WH) fold, which is sufficient for binding to the constitutive decay element (CDE) in the Tnf 3' UTR. The crystal structure of the ROQ domain in complex with a prototypical CDE RNA stem-loop reveals tight recognition of the RNA stem and its triloop. Surprisingly, roquin uses mainly non-sequence-specific contacts to the RNA, thus suggesting a relaxed CDE consensus and implicating a broader spectrum of target mRNAs than previously anticipated. Consistently with this, NMR and binding experiments with CDE-like stem-loops together with cell-based assays confirm roquin-dependent regulation of relaxed CDE consensus motifs in natural 3' UTRs.