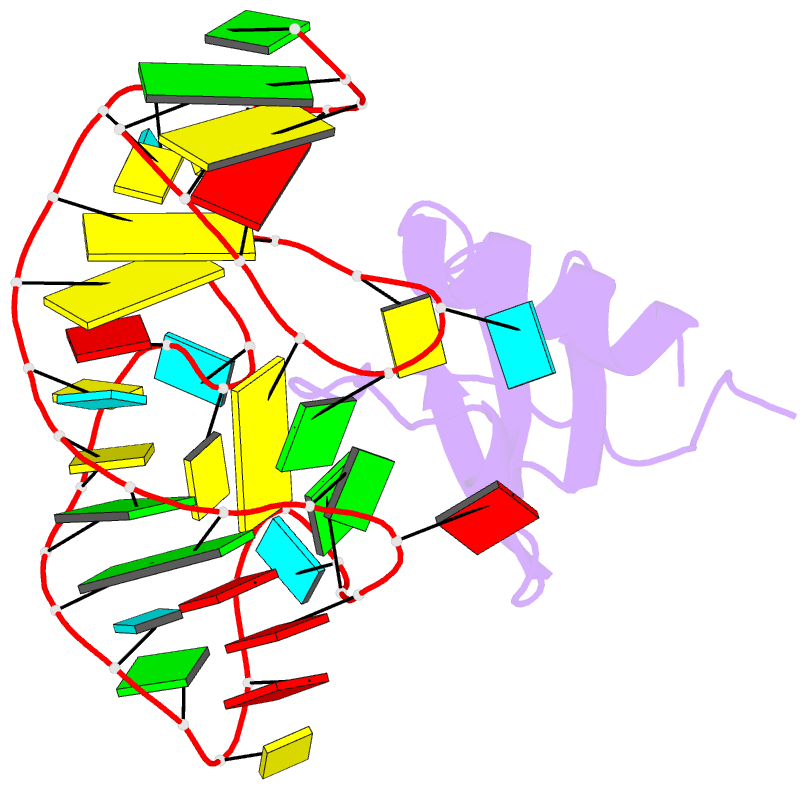
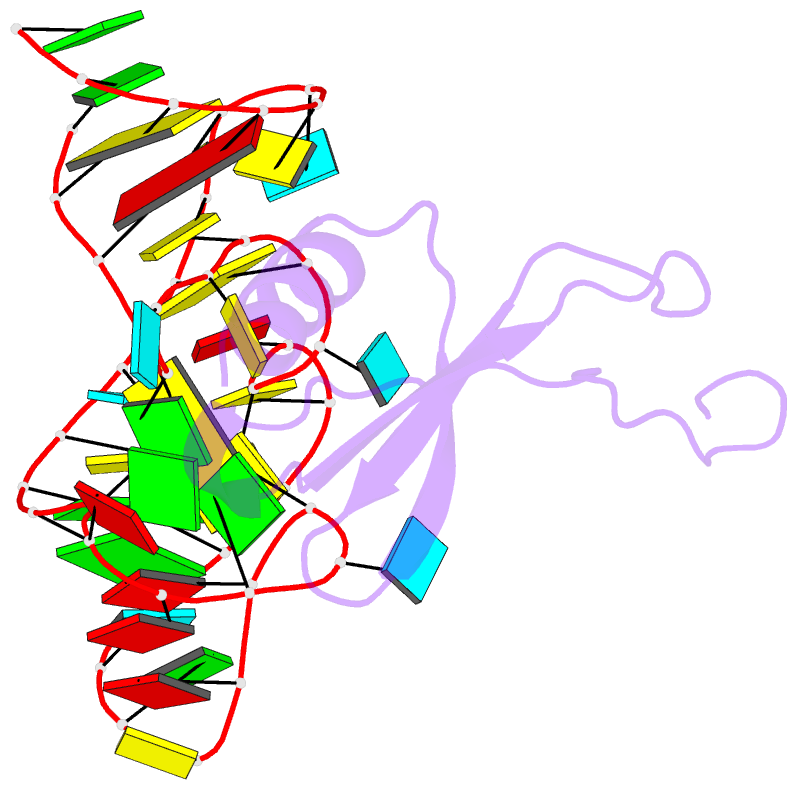
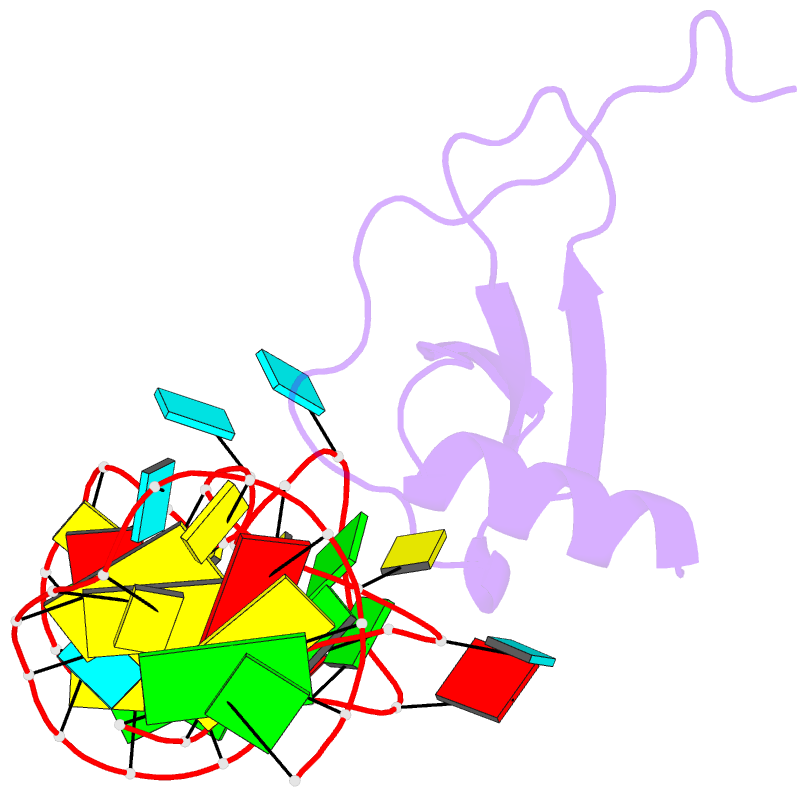
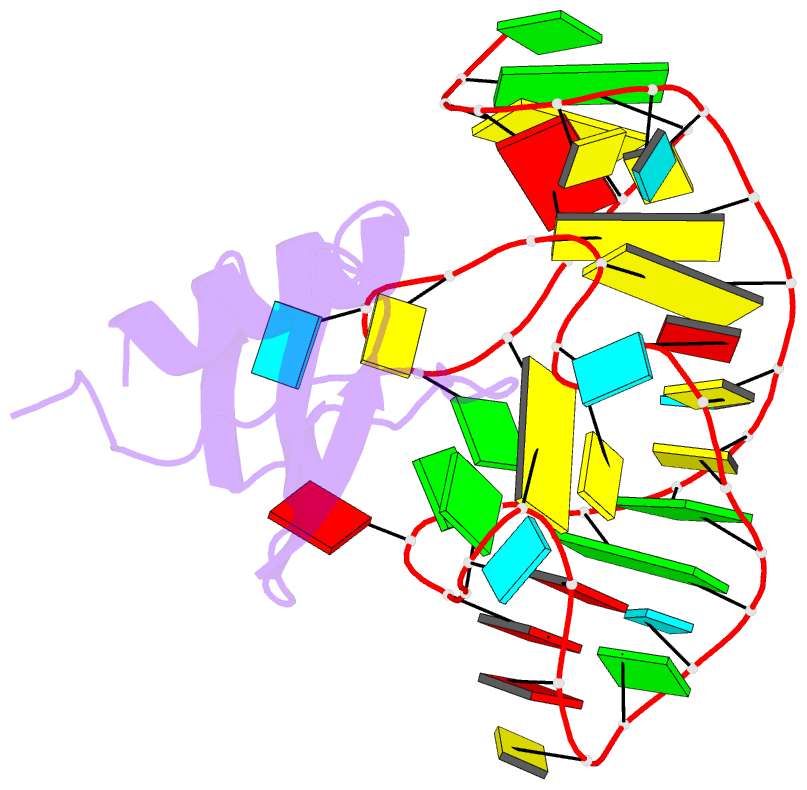
Summary information and primary citation
- PDB-id
- 4r8i; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- cytokine-RNA
- Method
- X-ray (2.05 Å)
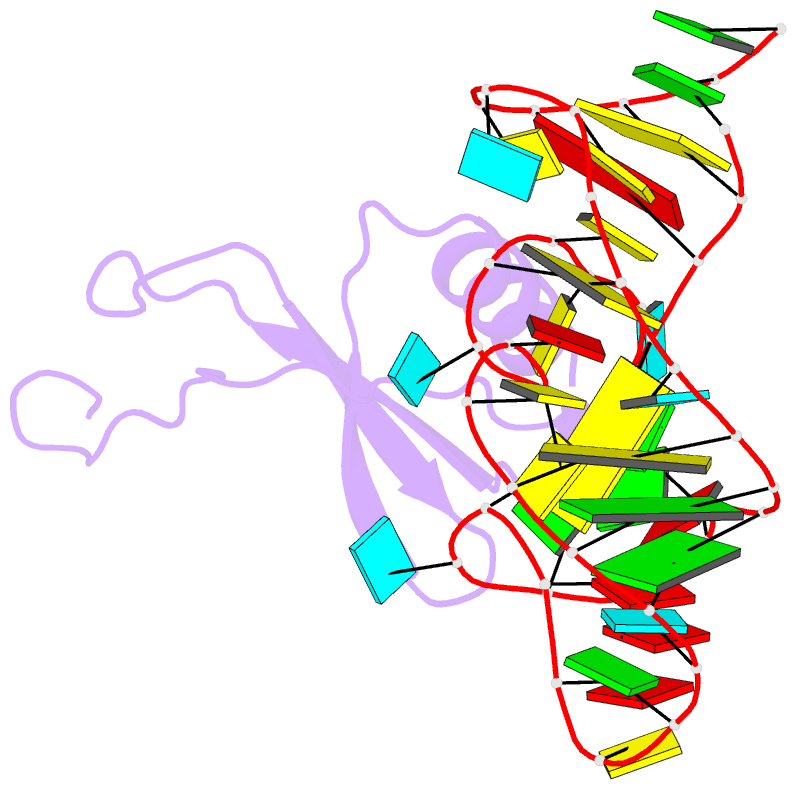
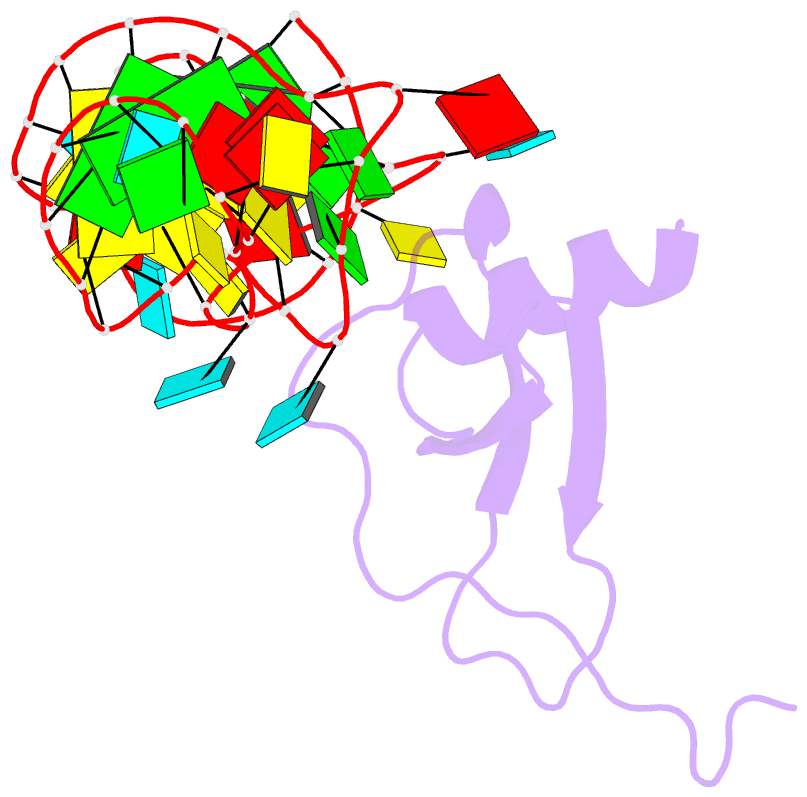
- Summary
- High resolution structure of a mirror-image RNA oligonucleotide aptamer in complex with the chemokine ccl2
- Reference
- Oberthur D, Achenbach J, Gabdulkhakov A, Buchner K, Maasch C, Falke S, Rehders D, Klussmann S, Betzel C (2015): "Crystal structure of a mirror-image L-RNA aptamer (Spiegelmer) in complex with the natural L-protein target CCL2." Nat Commun, 6, 6923. doi: 10.1038/ncomms7923.
- Abstract
- We report the crystal structure of a 40 mer mirror-image RNA oligonucleotide completely built from nucleotides of the non-natural L-chirality in complex with the pro-inflammatory chemokine L-CLL2 (monocyte chemoattractant protein-1), a natural protein composed of regular L-amino acids. The L-oligonucleotide is an L-aptamer (a Spiegelmer) identified to bind L-CCL2 with high affinity, thereby neutralizing the chemokine's activity. CCL2 plays a key role in attracting and positioning monocytes; its overexpression in several inflammatory diseases makes CCL2 an interesting pharmacological target. The PEGylated form of the L-aptamer, NOX-E36 (emapticap pegol), already showed promising efficacy in clinical Phase II studies conducted in diabetic nephropathy patients. The structure of the L-oligonucleotide[Symbol: see text]L-protein complex was solved and refined to 2.05 Å. It unveils the L-aptamer's intramolecular contacts and permits a detailed analysis of its structure-function relationship. Furthermore, the analysis of the intermolecular drug-target interactions reveals insight into the selectivity of the L-aptamer for certain related chemokines.