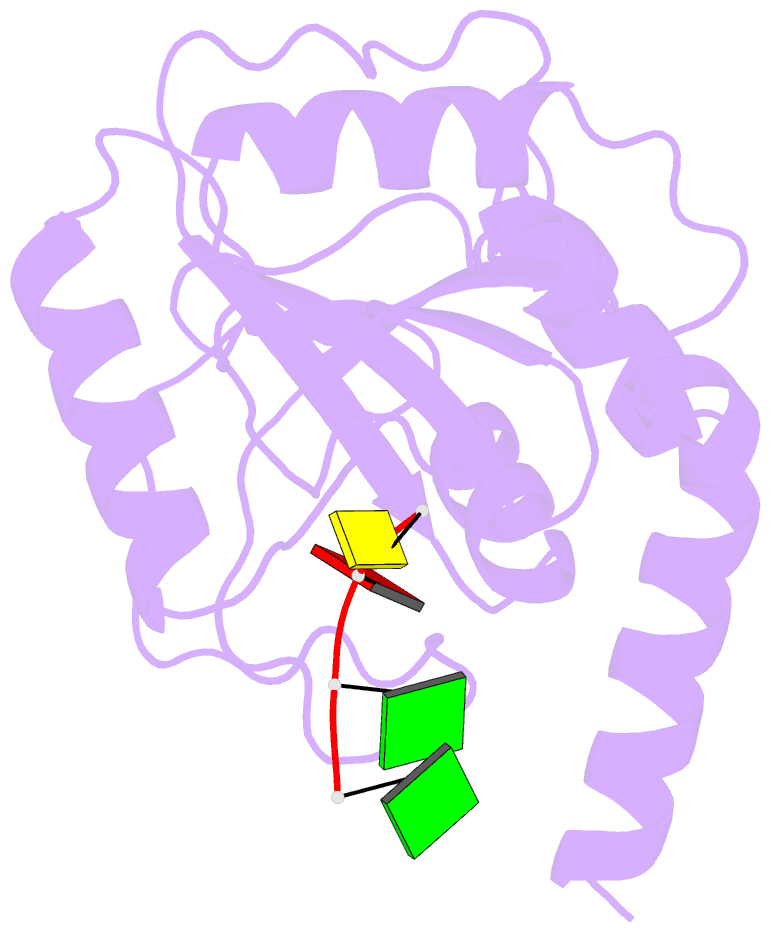
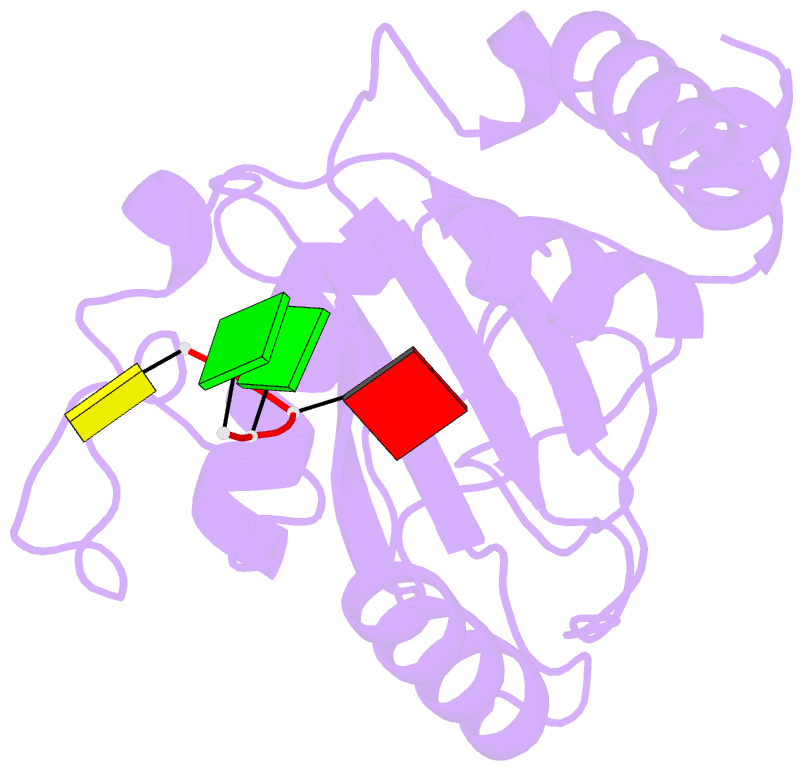
Summary information and primary citation
- PDB-id
- 4rcj; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (1.6 Å)
- Summary
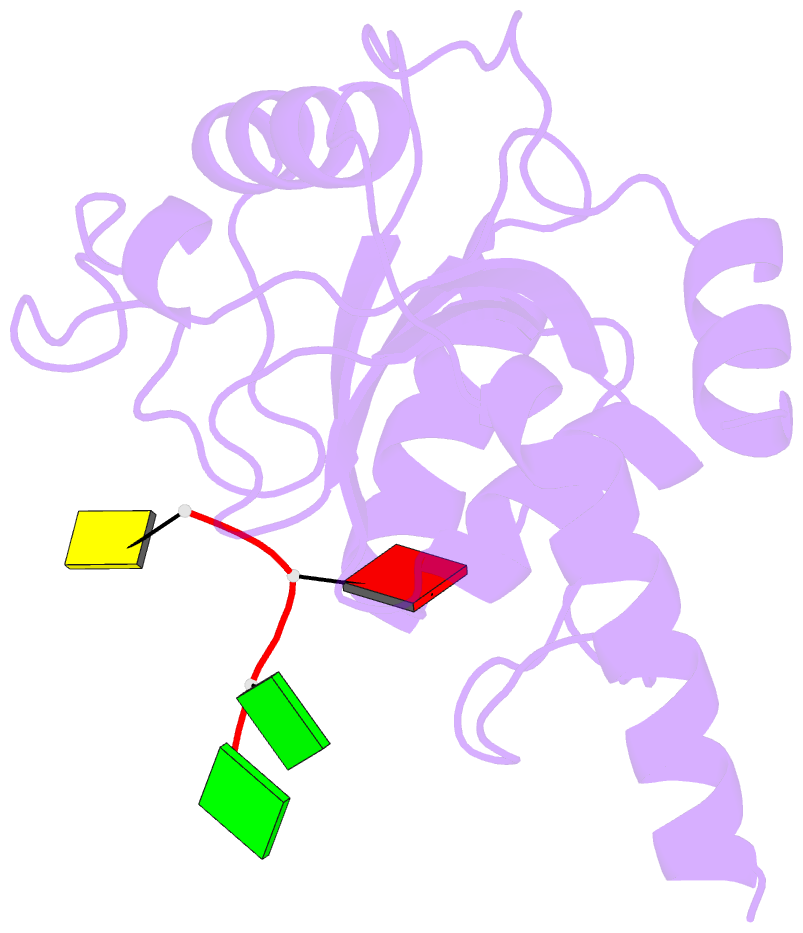
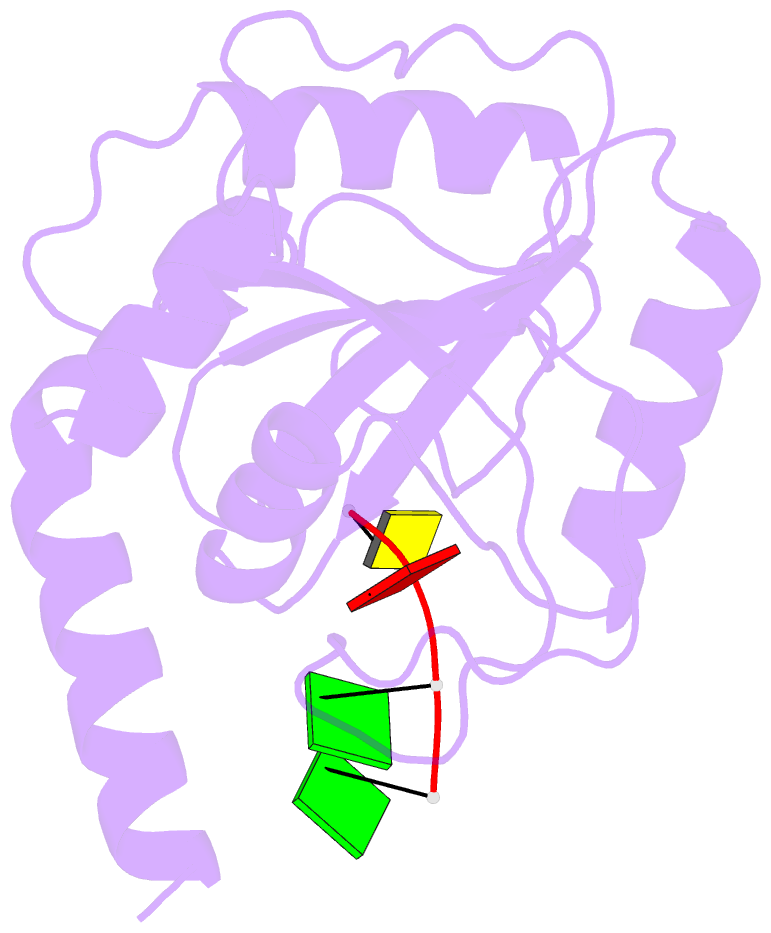
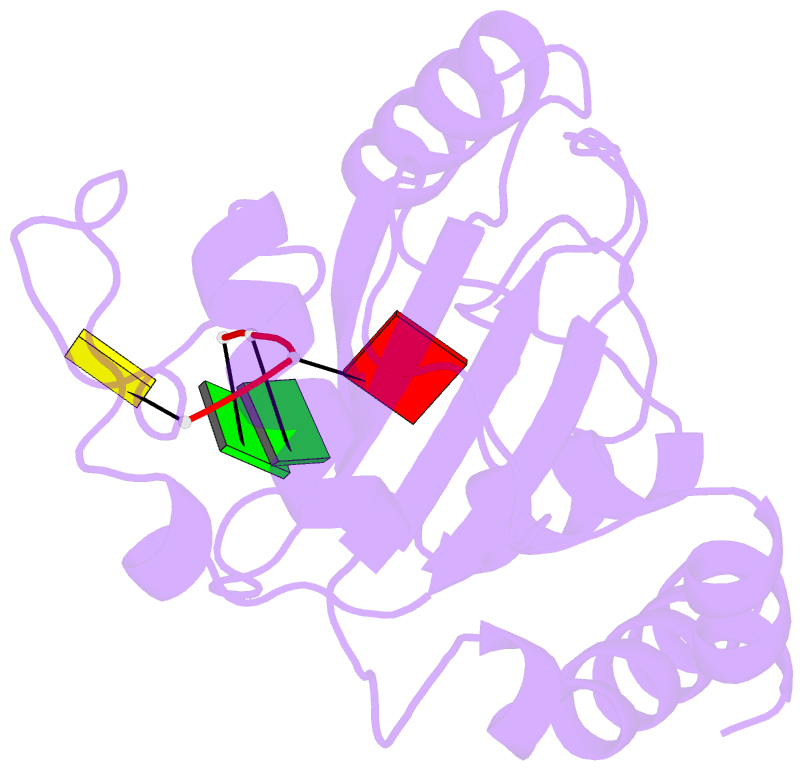
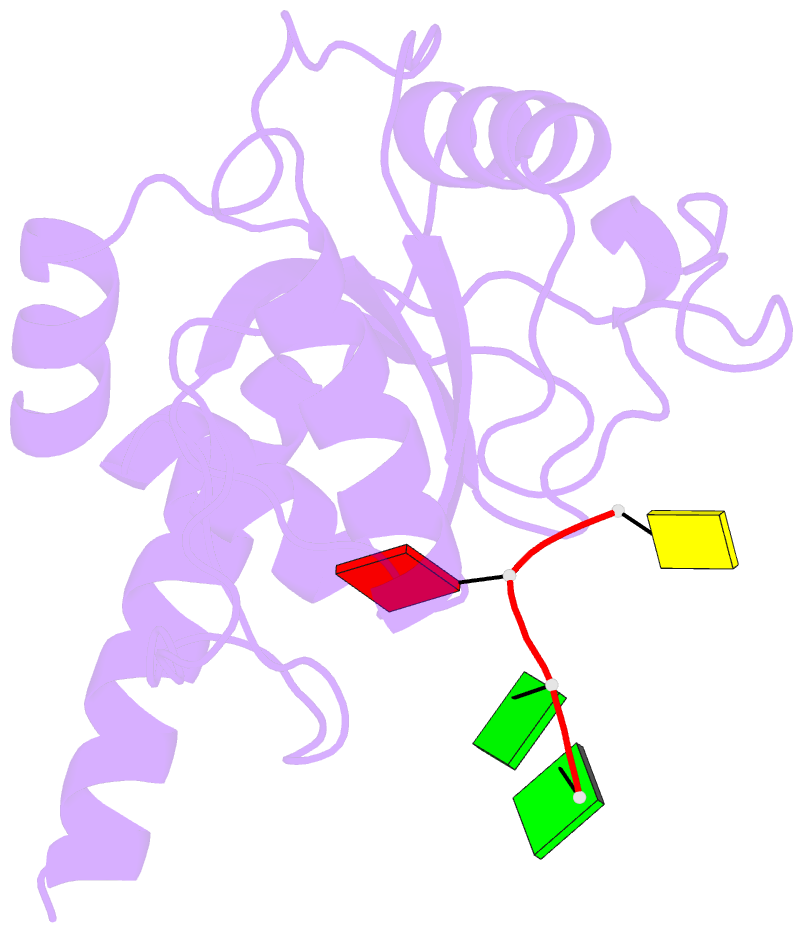
- Crystal structure of ythdf1 yth domain in complex with 5mer m6a RNA
- Reference
- Xu C, Liu K, Ahmed H, Loppnau P, Schapira M, Min J (2015): "Structural Basis for the Discriminative Recognition of N6-Methyladenosine RNA by the Human YT521-B Homology Domain Family of Proteins." J.Biol.Chem., 290, 24902-24913. doi: 10.1074/jbc.M115.680389.
- Abstract
- N(6)-Methyladenosine (m(6)A) is the most abundant internal modification in RNA and is specifically recognized by YT521-B homology (YTH) domain-containing proteins. Recently we reported that YTHDC1 prefers guanosine and disfavors adenosine at the position preceding the m(6)A nucleotide in RNA and preferentially binds to the GG(m(6)A)C sequence. Now we systematically characterized the binding affinities of the YTH domains of three other human proteins and yeast YTH domain protein Pho92 and determined the crystal structures of the YTH domains of human YTHDF1 and yeast Pho92 in complex with a 5-mer m(6)A RNA, respectively. Our binding and structural data revealed that the YTH domain used a conserved aromatic cage to recognize m(6)A. Nevertheless, none of these YTH domains, except YTHDC1, display sequence selectivity at the position preceding the m(6)A modification. Structural comparison of these different YTH domains revealed that among those, only YTHDC1 harbors a distinctly selective binding pocket for the nucleotide preceding the m(6)A nucleotide.