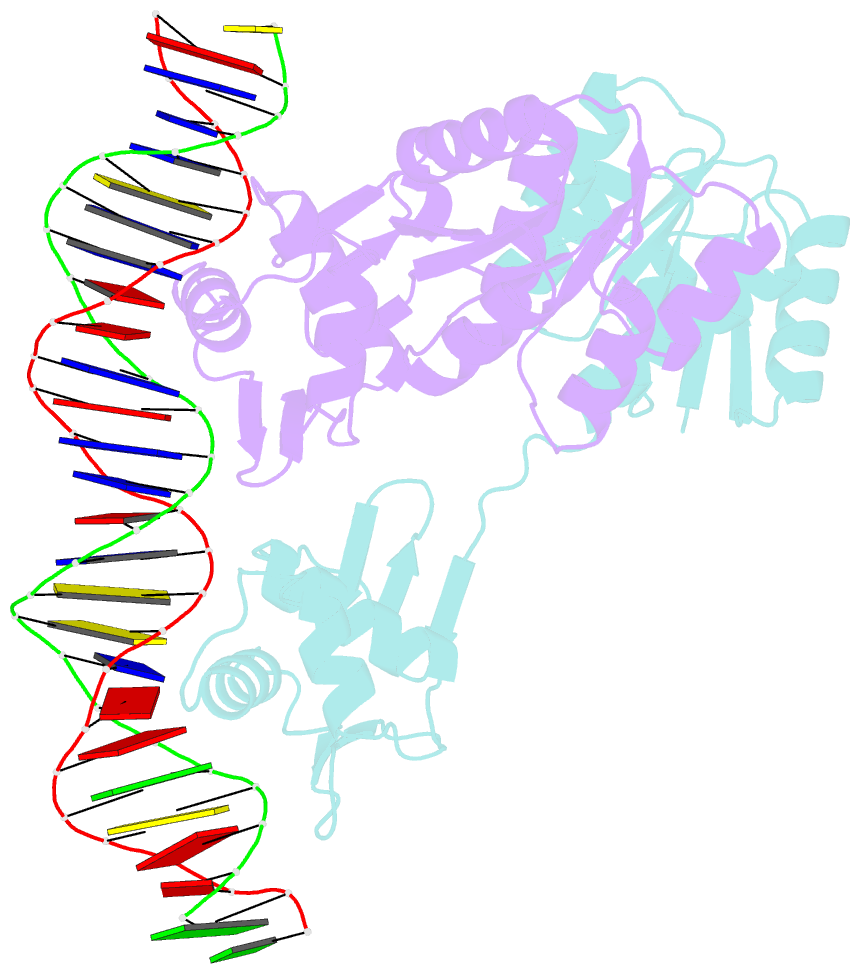
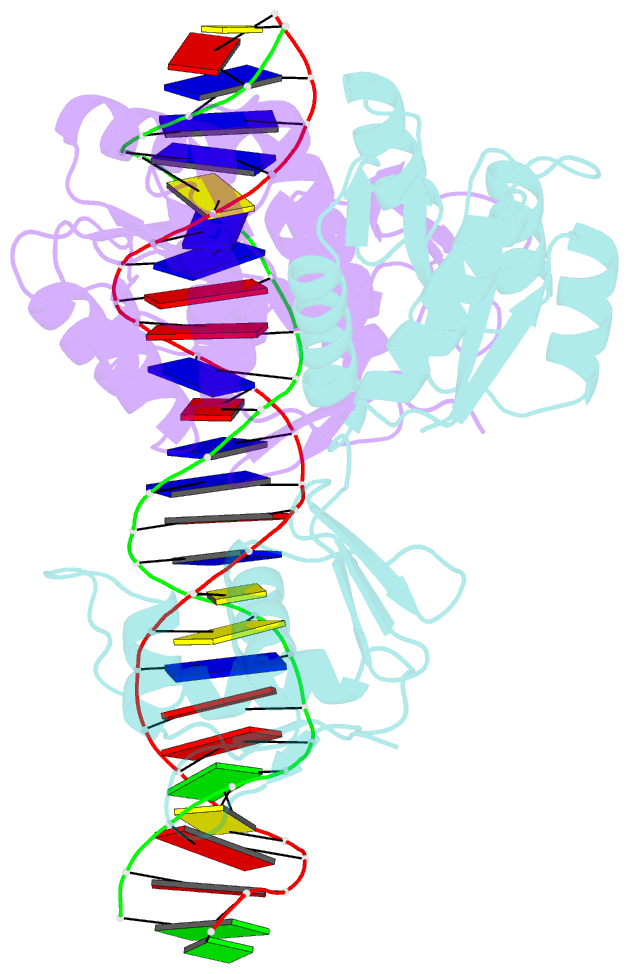
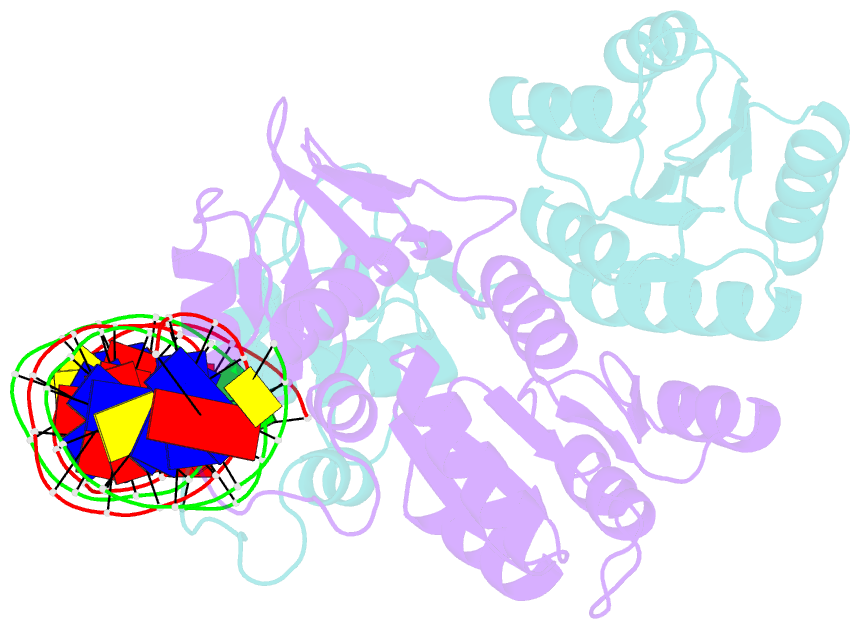
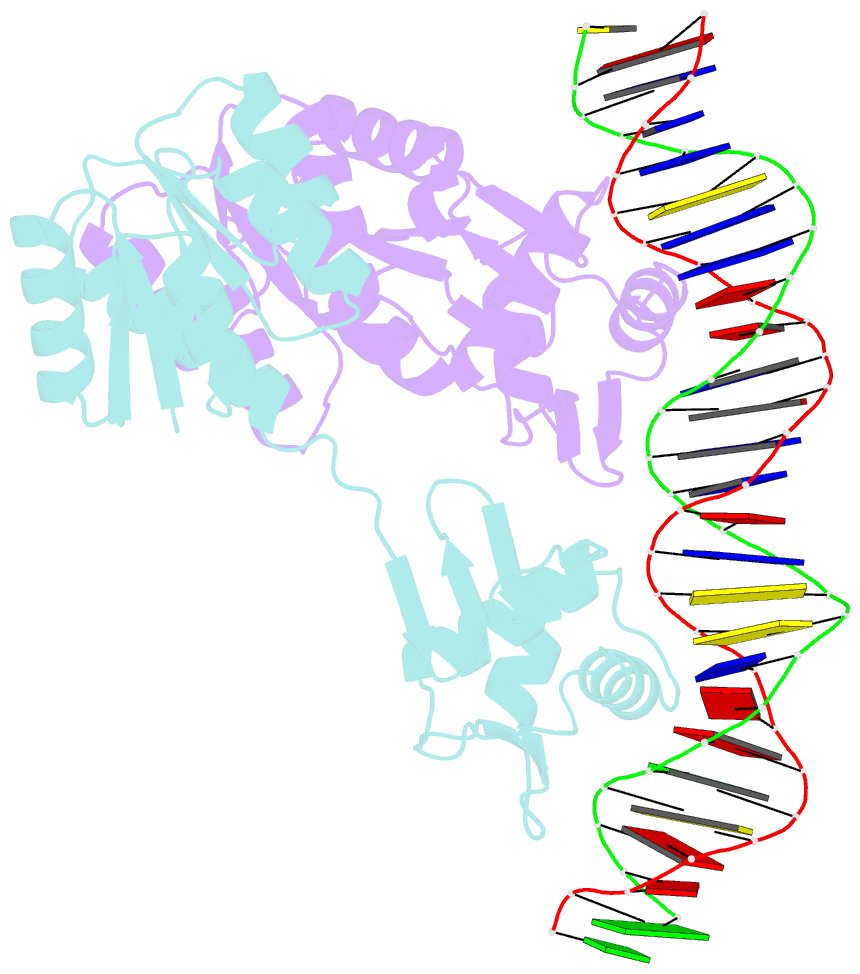
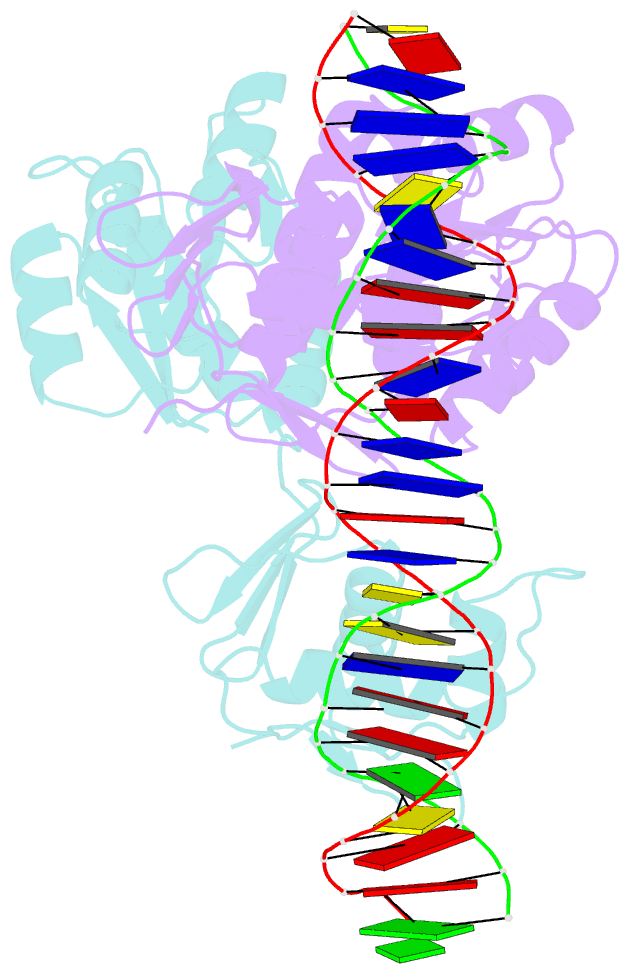
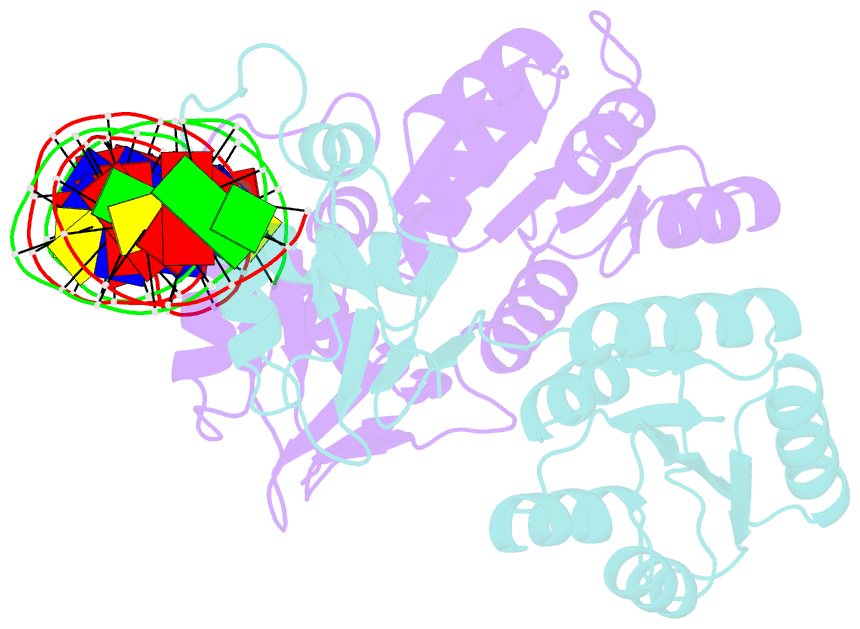
Summary information and primary citation
- PDB-id
- 4s05; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (3.8 Å)
- Summary
- Crystal structure of klebsiella pneumoniae pmra in complex with pmra box DNA
- Reference
- Lou YC, Weng TH, Li YC, Kao YF, Lin WF, Peng HL, Chou SH, Hsiao CD, Chen C (2015): "Structure and dynamics of polymyxin-resistance-associated response regulator PmrA in complex with promoter DNA." Nat Commun, 6, 8838. doi: 10.1038/ncomms9838.
- Abstract
- PmrA, an OmpR/PhoB family response regulator, manages genes for antibiotic resistance. Phosphorylation of OmpR/PhoB response regulator induces the formation of a symmetric dimer in the N-terminal receiver domain (REC), promoting two C-terminal DNA-binding domains (DBDs) to recognize promoter DNA to elicit adaptive responses. Recently, determination of the KdpE-DNA complex structure revealed an REC-DBD interface in the upstream protomer that may be necessary for transcription activation. Here, we report the 3.2-Å-resolution crystal structure of the PmrA-DNA complex, which reveals a similar yet different REC-DBD interface. However, NMR studies show that in the DNA-bound state, two domains tumble separately and an REC-DBD interaction is transiently populated in solution. Reporter gene analyses of PmrA variants with altered interface residues suggest that the interface is not crucial for supporting gene expression. We propose that REC-DBD interdomain dynamics and the DBD-DBD interface help PmrA interact with RNA polymerase holoenzyme to activate downstream gene transcription.