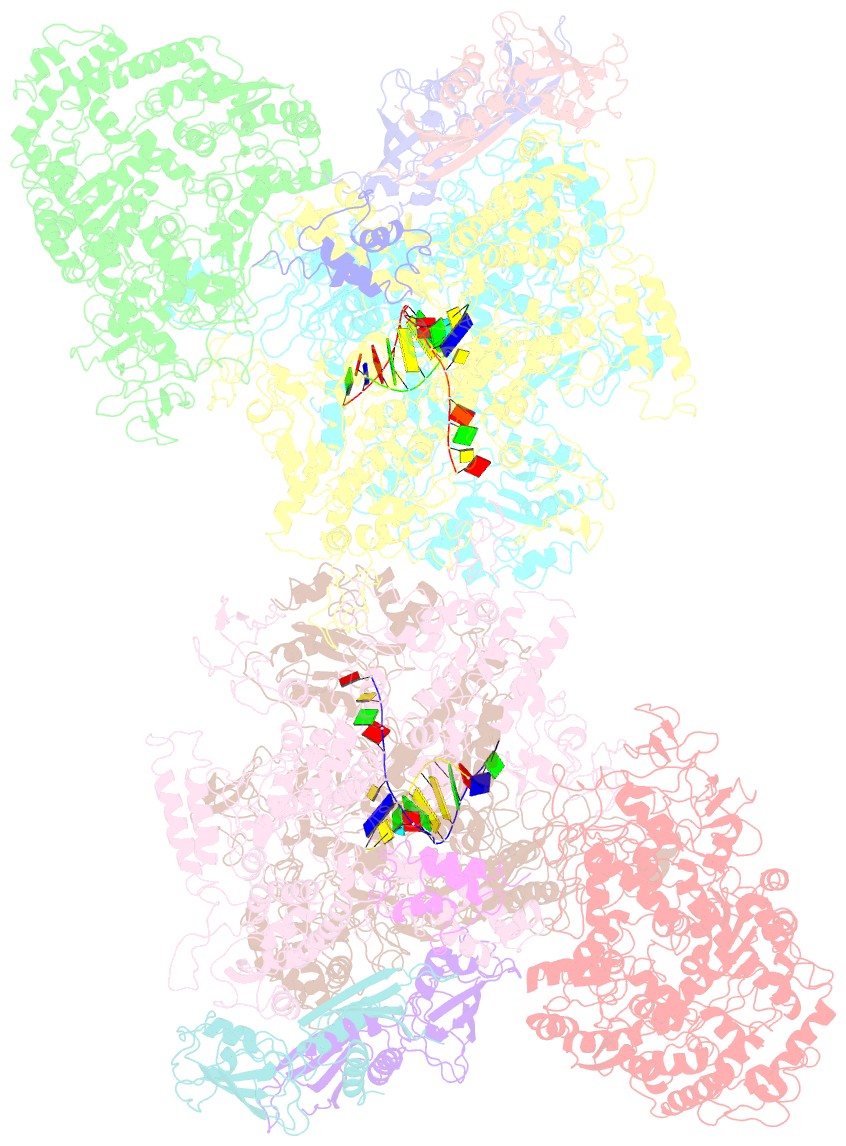
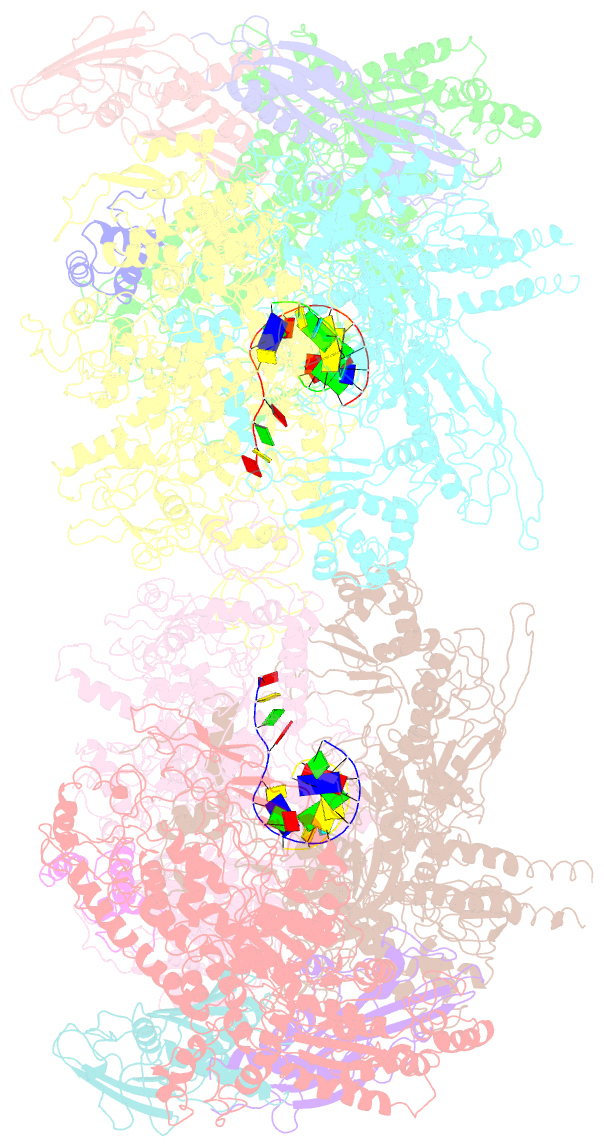
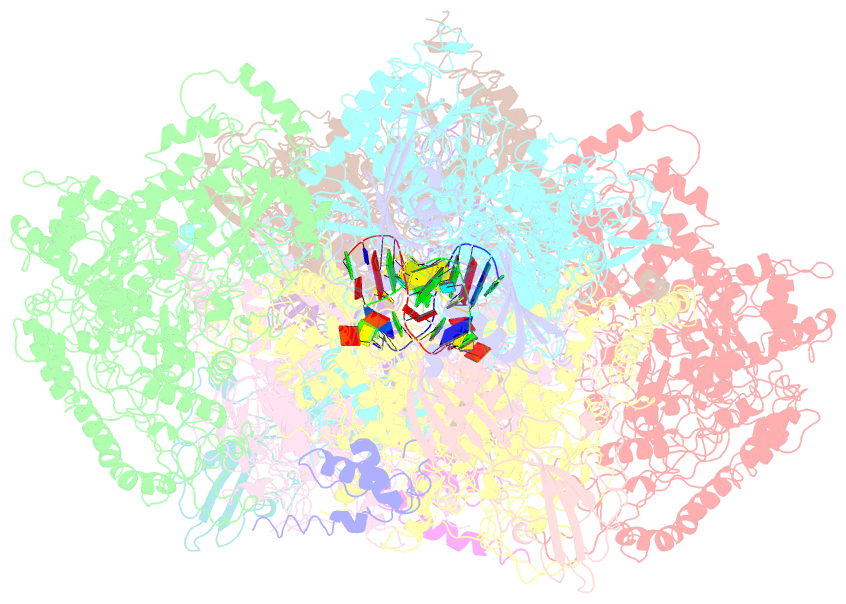
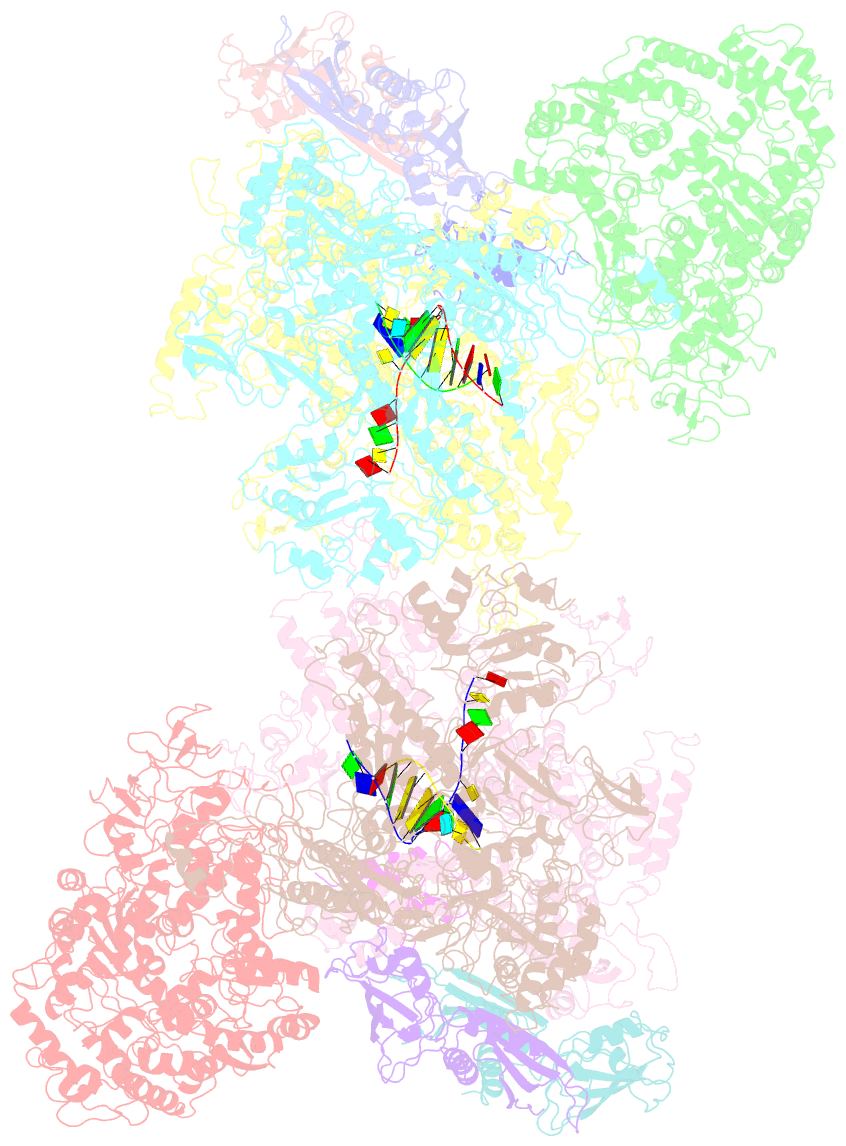
Summary information and primary citation
- PDB-id
- 4s20; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA-RNA
- Method
- X-ray (4.7 Å)
- Summary
- Structural basis for transcription reactivation by rapa
- Reference
- Liu B, Zuo Y, Steitz TA (2015): "Structural basis for transcription reactivation by RapA." Proc.Natl.Acad.Sci.USA, 112, 2006-2010. doi: 10.1073/pnas.1417152112.
- Abstract
- RNA polymerase (RNAP) loses activity during transcription as it stalls at various inactive states due to erratic translocation. Reactivation of these stalled RNAPs is essential for efficient RNA synthesis. Here we report a 4.7-Å resolution crystal structure of the Escherichia coli RNAP core enzyme in complex with ATPase RapA that is involved in reactivating stalled RNAPs. The structure reveals that RapA binds at the RNA exit channel of the RNAP and makes the channel unable to accommodate the formation of an RNA hairpin. The orientation of RapA on the RNAP core complex suggests that RapA uses its ATPase activity to propel backward translocation of RNAP along the DNA template in an elongation complex. This structure provides insights into the reactivation of stalled RNA polymerases and helps support ATP-driven backward translocation as a general mechanism for transcriptional regulation.