Summary information and primary citation
- PDB-id
- 4tmu; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.4 Å)
- Summary
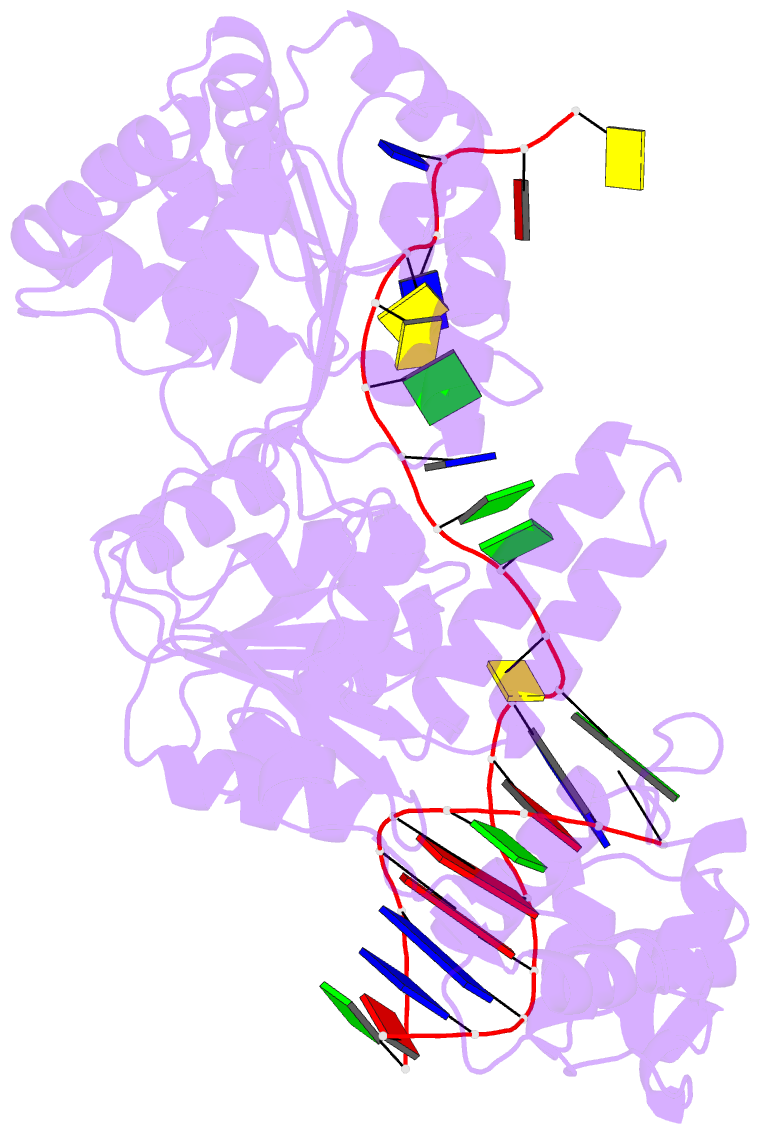
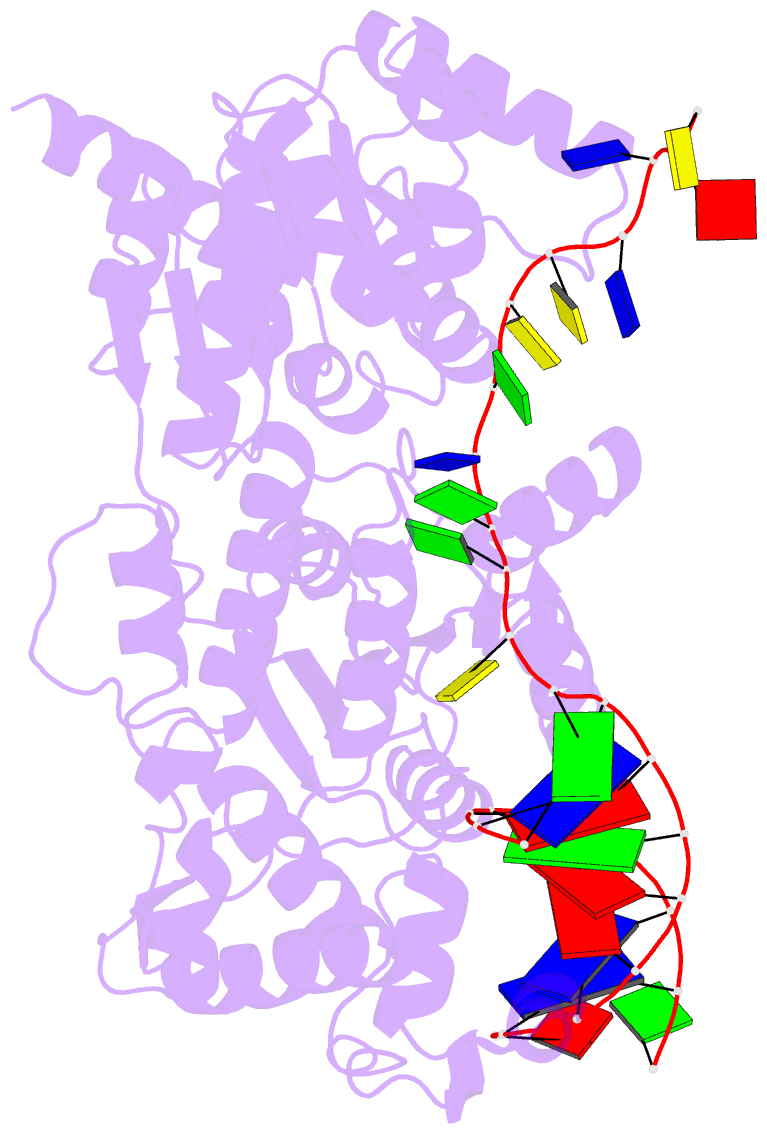
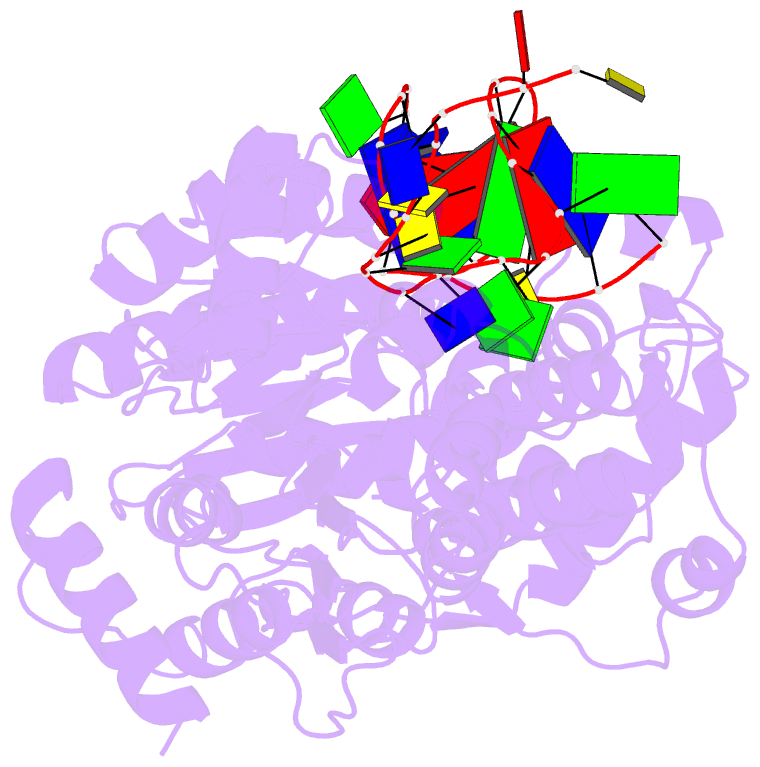
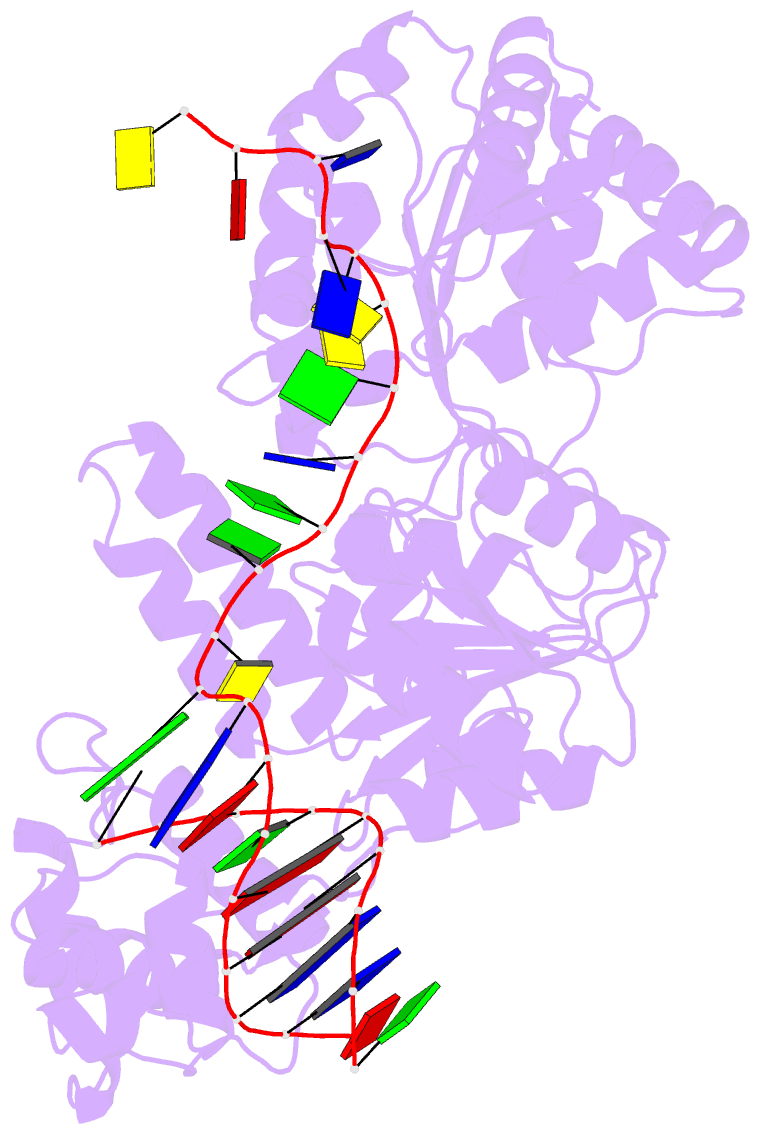
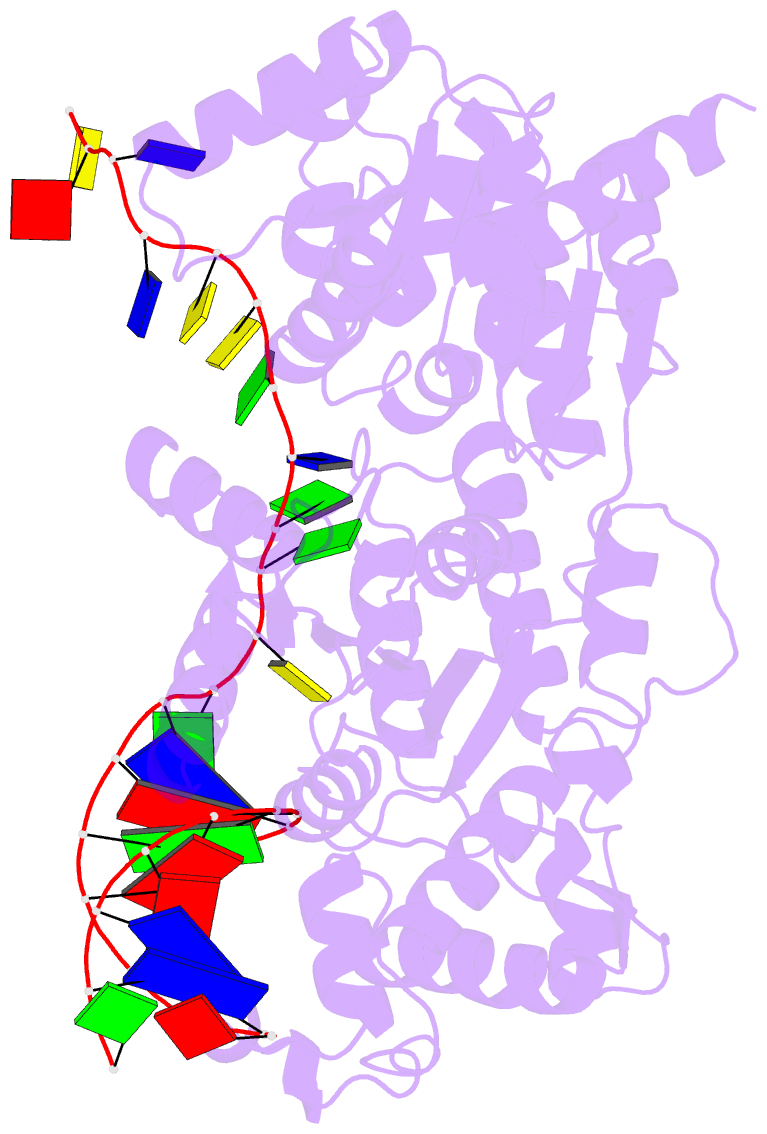
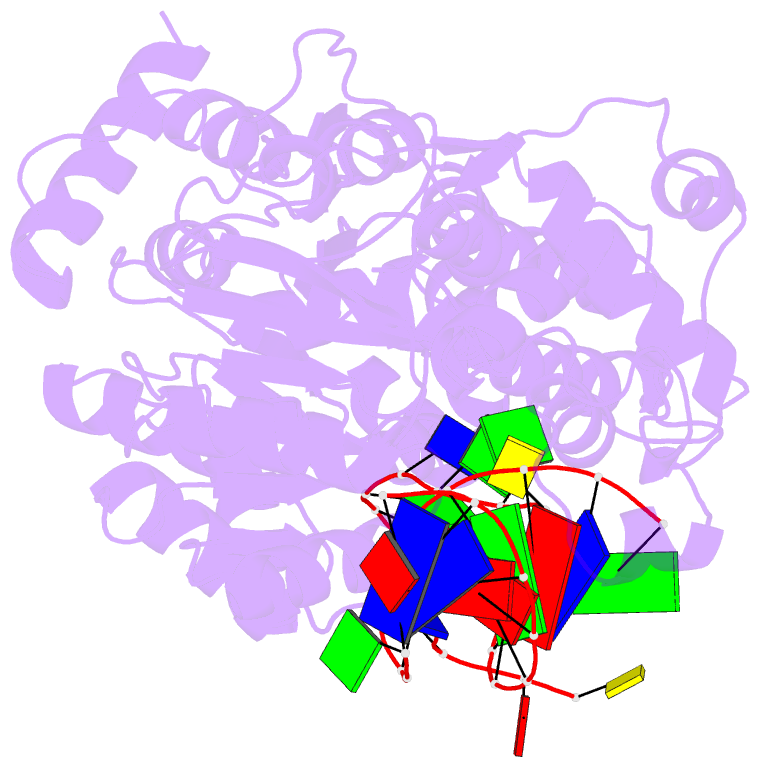
- Crystal structure of recq catalytic core from c. sakazakii bound to DNA
- Reference
- Manthei KA, Hill MC, Burke JE, Butcher SE, Keck JL (2015): "Structural mechanisms of DNA binding and unwinding in bacterial RecQ helicases." Proc.Natl.Acad.Sci.USA, 112, 4292-4297. doi: 10.1073/pnas.1416746112.
- Abstract
- RecQ helicases unwind remarkably diverse DNA structures as key components of many cellular processes. How RecQ enzymes accommodate different substrates in a unified mechanism that couples ATP hydrolysis to DNA unwinding is unknown. Here, the X-ray crystal structure of the Cronobacter sakazakii RecQ catalytic core domain bound to duplex DNA with a 3' single-stranded extension identifies two DNA-dependent conformational rearrangements: a winged-helix domain pivots ∼90° to close onto duplex DNA, and a conserved aromatic-rich loop is remodeled to bind ssDNA. These changes coincide with a restructuring of the RecQ ATPase active site that positions catalytic residues for ATP hydrolysis. Complex formation also induces a tight bend in the DNA and melts a portion of the duplex. This bending, coupled with translocation, could provide RecQ with a mechanism for unwinding duplex and other DNA structures.