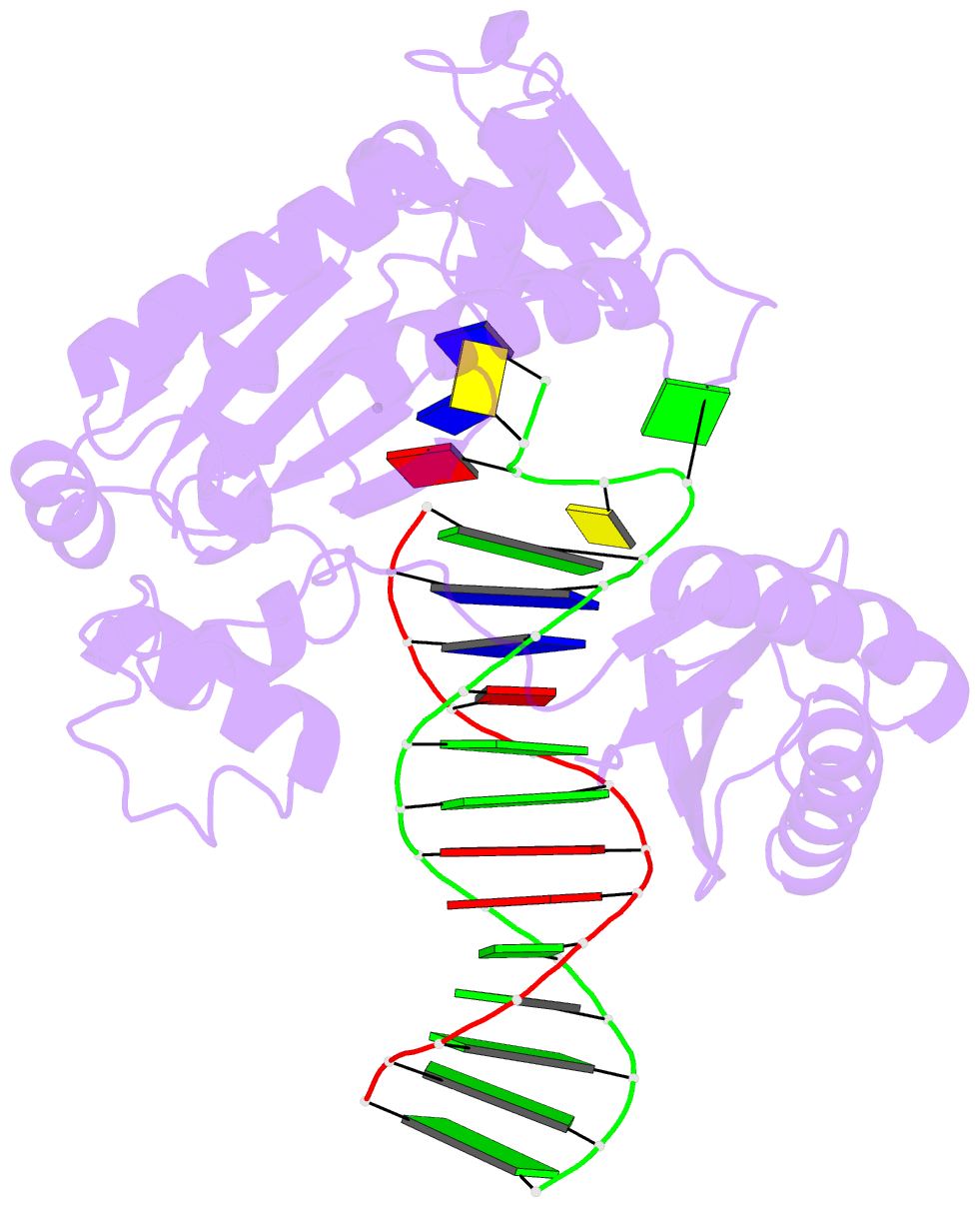
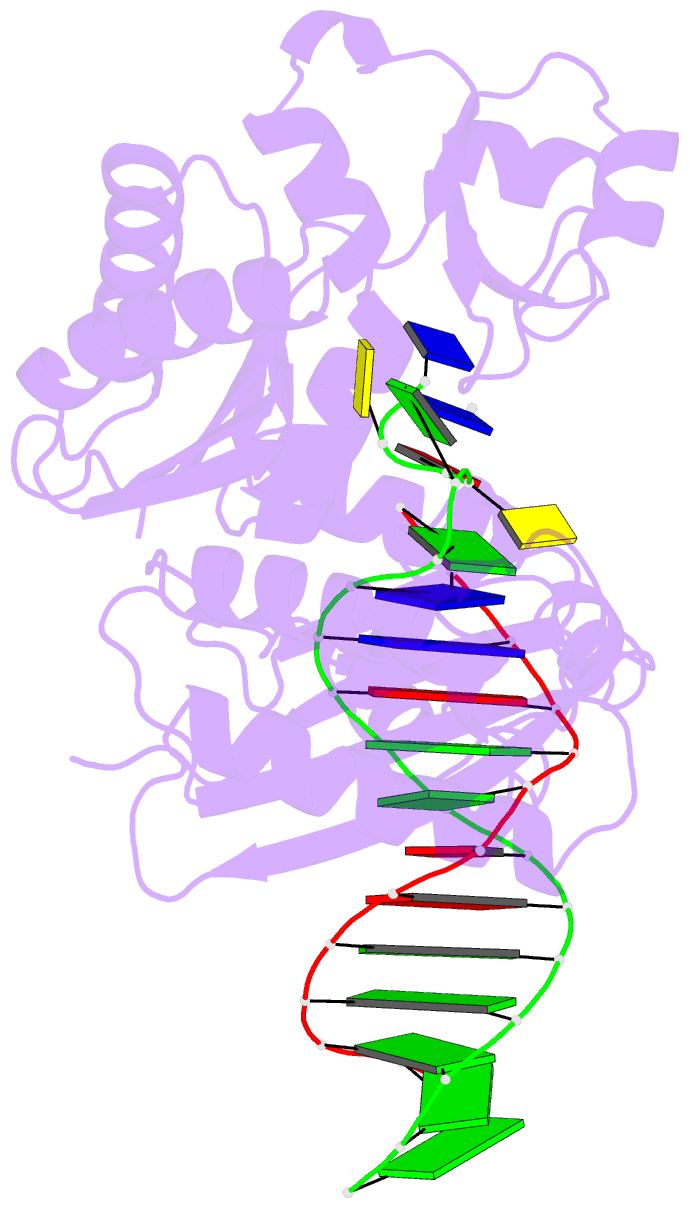
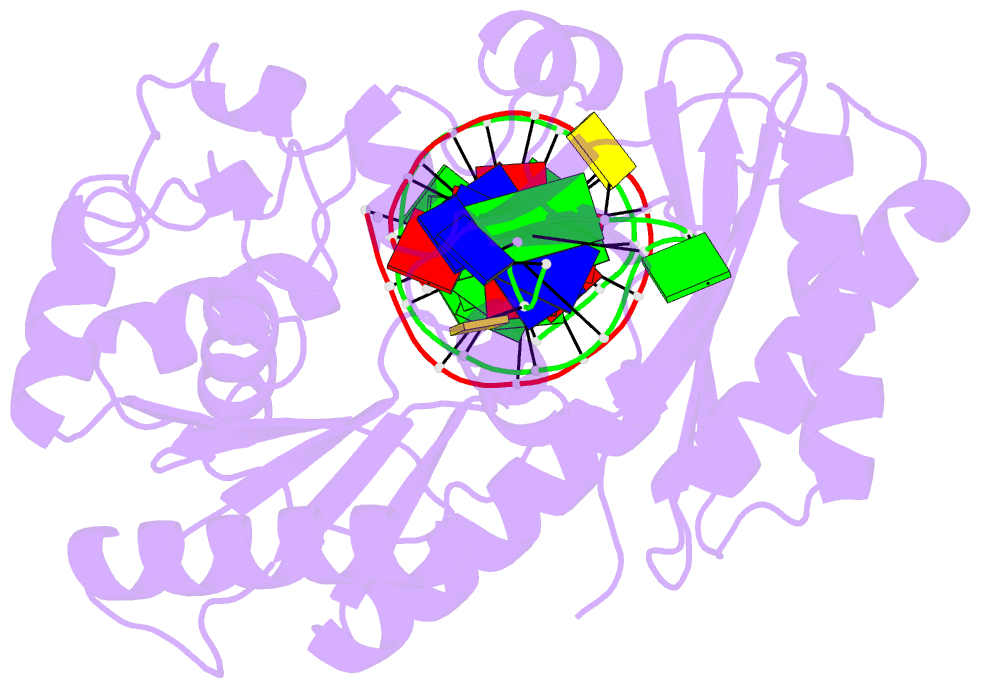
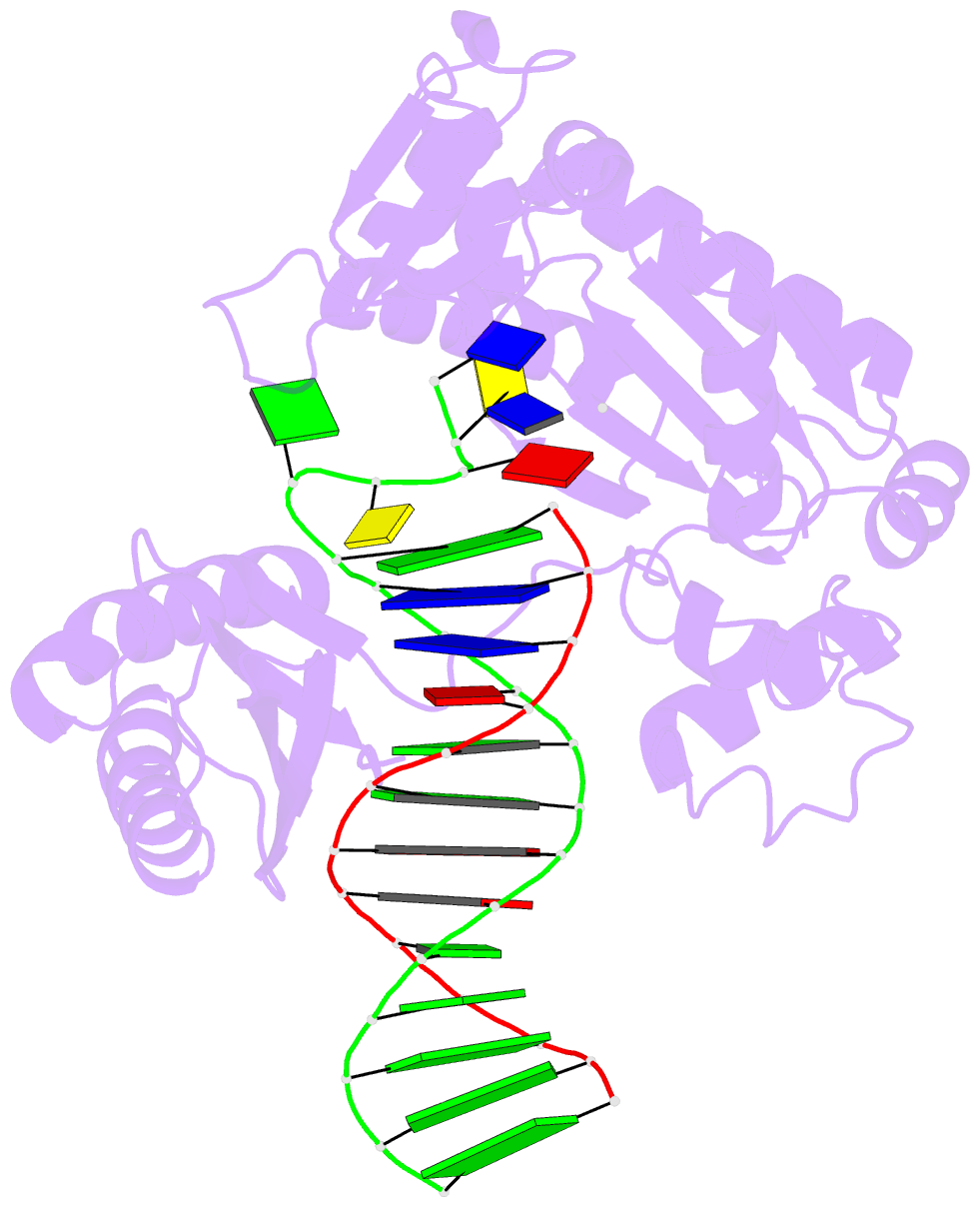
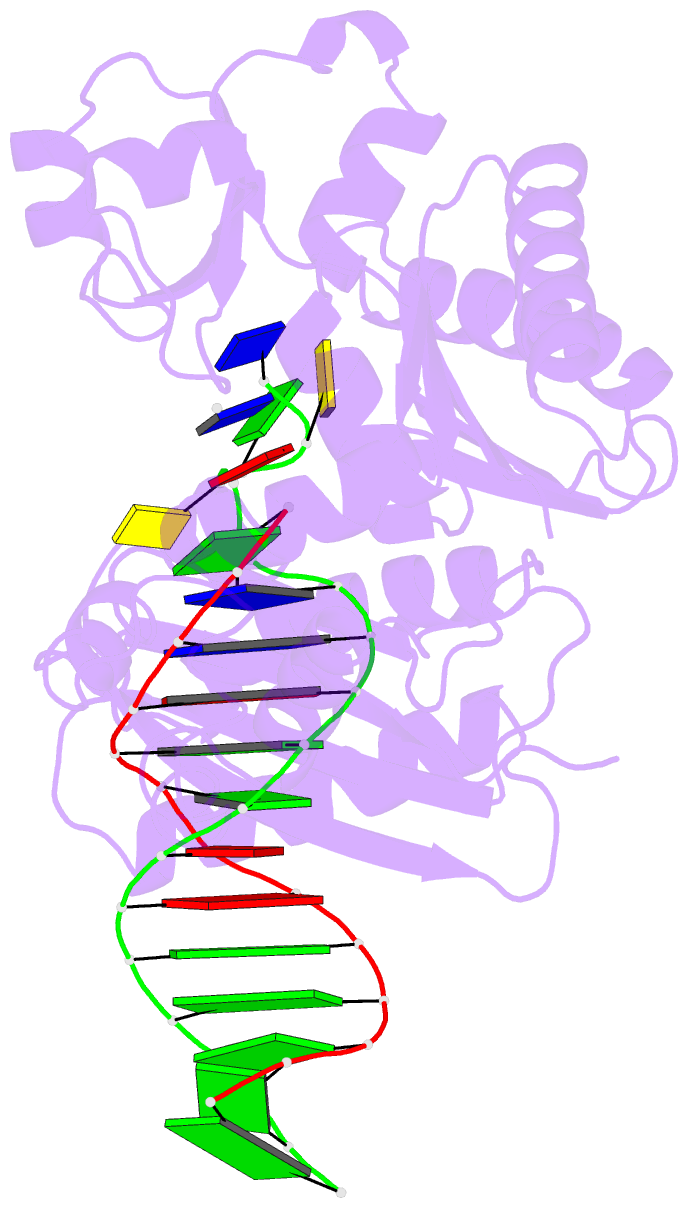
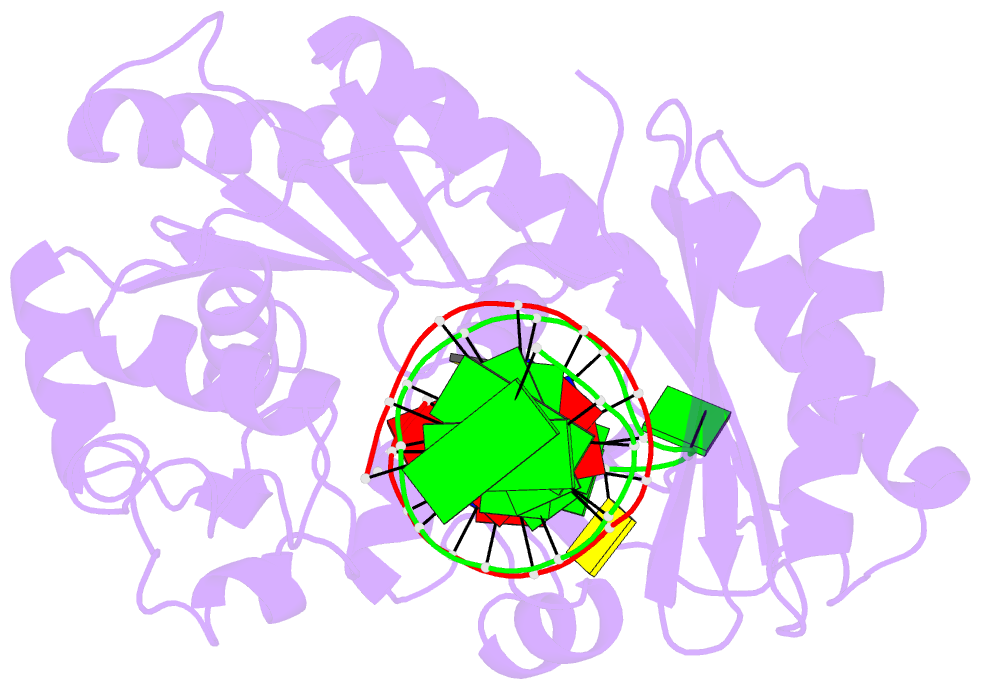
Summary information and primary citation
- PDB-id
- 4tqr; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (1.58 Å)
- Summary
- Ternary complex of y-family DNA polymerase dpo4 with (5's)-8,5'-cyclo-2'-deoxyguanosine and dttp
- Reference
- Xu W, Ouellette AM, Wawrzak Z, Shriver SJ, Anderson SM, Zhao L (2015): "Kinetic and Structural Mechanisms of (5'S)-8,5'-Cyclo-2'-deoxyguanosine-Induced DNA Replication Stalling." Biochemistry, 54, 639-651. doi: 10.1021/bi5014936.
- Abstract
- The (5'S)-8,5'-cyclo-2'-deoxyguanosine (S-cdG) lesion is produced from reactions of DNA with hydroxyl radicals generated from ionizing radiation or endogenous oxidative metabolisms. An elevated level of S-cdG has been detected in Xeroderma pigmentosum, Cockayne syndrome, breast cancer patients, and aged mice. S-dG blocks DNA replication and transcription in vitro and in human cells and produces mutant replication and transcription products in vitro and in vivo. Major cellular protection against S-dG includes nucleotide excision repair and translesion DNA synthesis. We used kinetic and crystallographic approaches to elucidate the molecular mechanisms of S-cdG-induced DNA replication stalling using model B-family Sulfolobus solfataricus P2 DNA polymerase B1 (Dpo1) and Y-family S. solfataricus P2 DNA polymerase IV (Dpo4). Dpo1 and Dpo4 inefficiently bypassed S-cdG with dCTP preferably incorporated and dTTP (for Dpo4) or dATP (for Dpo1) misincorporated. Pre-steady-state kinetics and crystallographic data mechanistically explained the low-efficiency bypass. For Dpo1, S-cdG attenuated Kd,dNTP,app and kpol. For Dpo4, the S-cdG-adducted duplex caused a 6-fold decrease in Dpo4:DNA binding affinity and significantly reduced the concentration of the productive Dpo4:DNA:dCTP complex. Consistent with the inefficient bypass, crystal structures of Dpo4:DNA(S-cdG):dCTP (error-free) and Dpo4:DNA(S-cdG):dTTP (error-prone) complexes were catalytically incompetent. In the Dpo4:DNA(S-cdG):dTTP structure, S-cdG induced a loop structure and caused an unusual 5'-template base clustering at the active site, providing the first structural evidence of the previously suggested template loop structure that can be induced by a cyclopurine lesion. Together, our results provided mechanistic insights into S-cdG-induced DNA replication stalling.