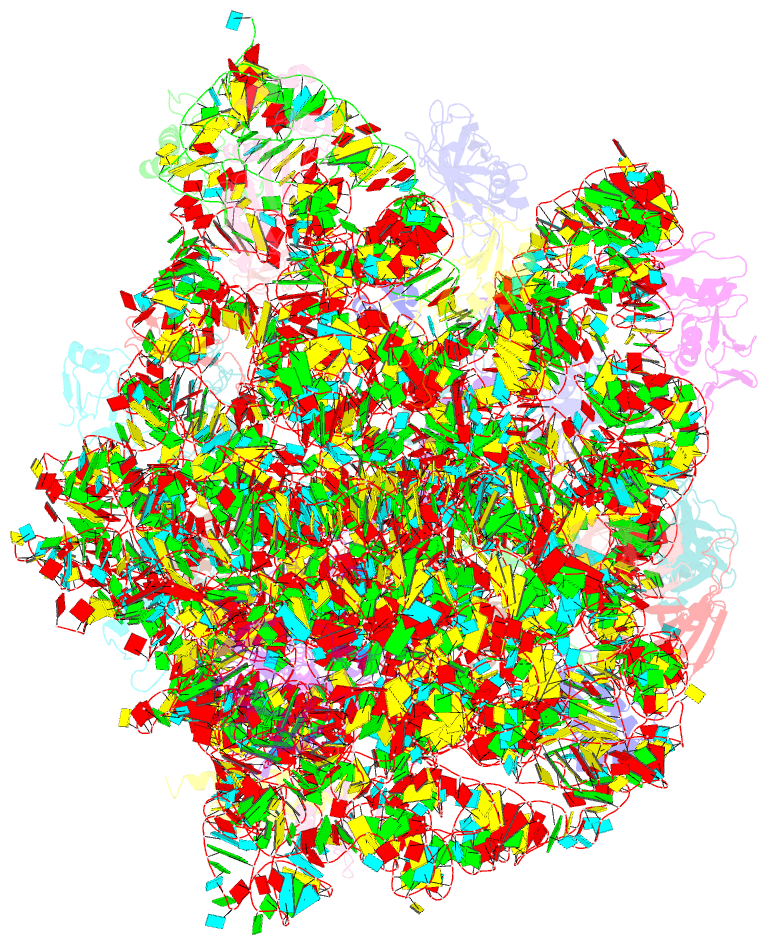
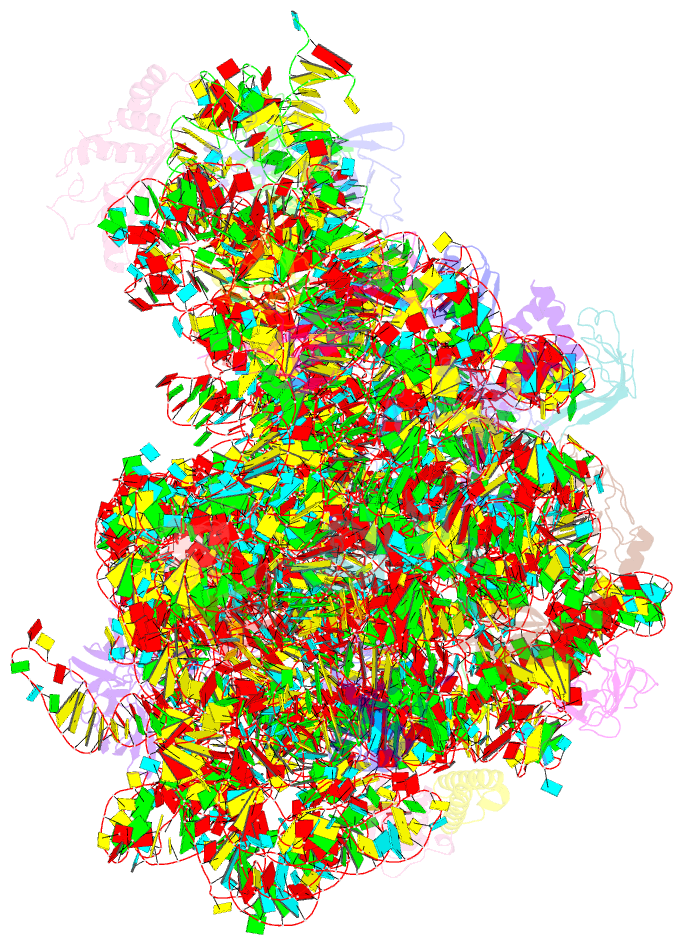
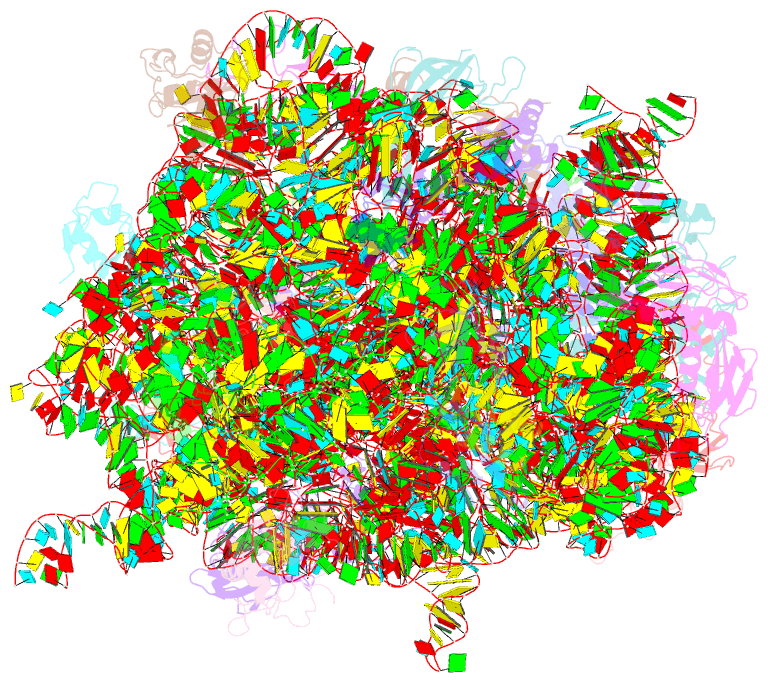
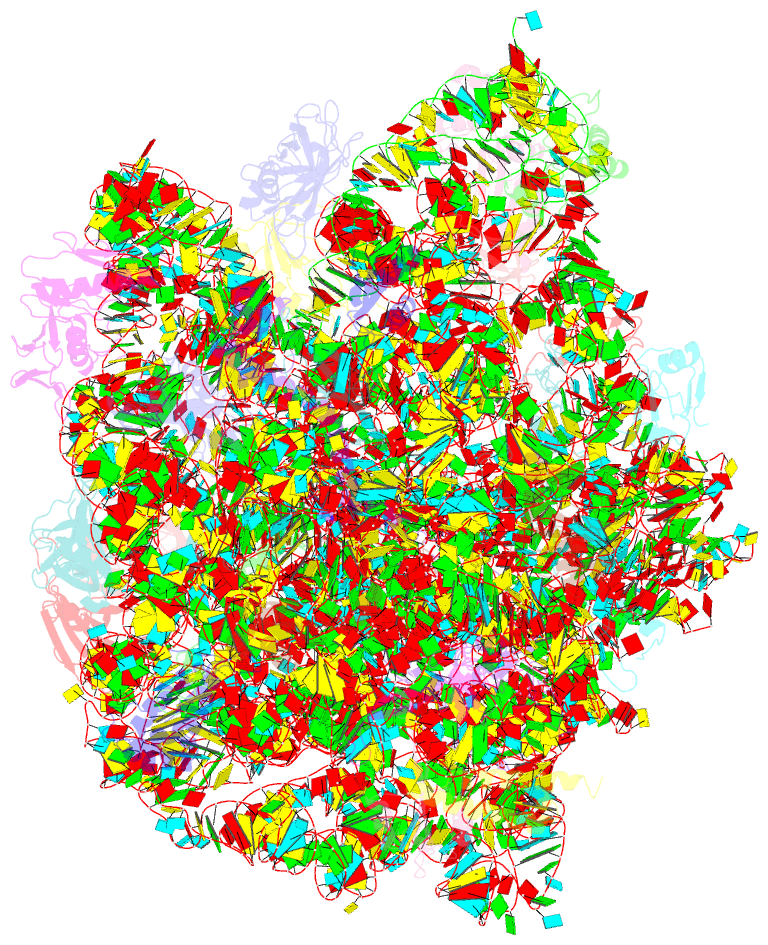
Summary information and primary citation
- PDB-id
- 4wfn; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- X-ray (3.54 Å)
- Summary
- Crystal structure of the large ribosomal subunit (50s) of deinococcus radiodurans containing a three residue insertion in l22 in complex with erythromycin
- Reference
- Wekselman I, Zimmerman E, Davidovich C, Belousoff M, Matzov D, Krupkin M, Rozenberg H, Bashan A, Friedlander G, Kjeldgaard J, Ingmer H, Lindahl L, Zengel JM, Yonath A (2017): "The Ribosomal Protein uL22 Modulates the Shape of the Protein Exit Tunnel." Structure, 25, 1233-1241.e3. doi: 10.1016/j.str.2017.06.004.
- Abstract
- Erythromycin is a clinically useful antibiotic that binds to an rRNA pocket in the ribosomal exit tunnel. Commonly, resistance to erythromycin is acquired by alterations of rRNA nucleotides that interact with the drug. Mutations in the β hairpin of ribosomal protein uL22, which is rather distal to the erythromycin binding site, also generate resistance to the antibiotic. We have determined the crystal structure of the large ribosomal subunit from Deinococcus radiodurans with a three amino acid insertion within the β hairpin of uL22 that renders resistance to erythromycin. The structure reveals a shift of the β hairpin of the mutated uL22 toward the interior of the exit tunnel, triggering a cascade of structural alterations of rRNA nucleotides that propagate to the erythromycin binding pocket. Our findings support recent studies showing that the interactions between uL22 and specific sequences within nascent chains trigger conformational rearrangements in the exit tunnel.