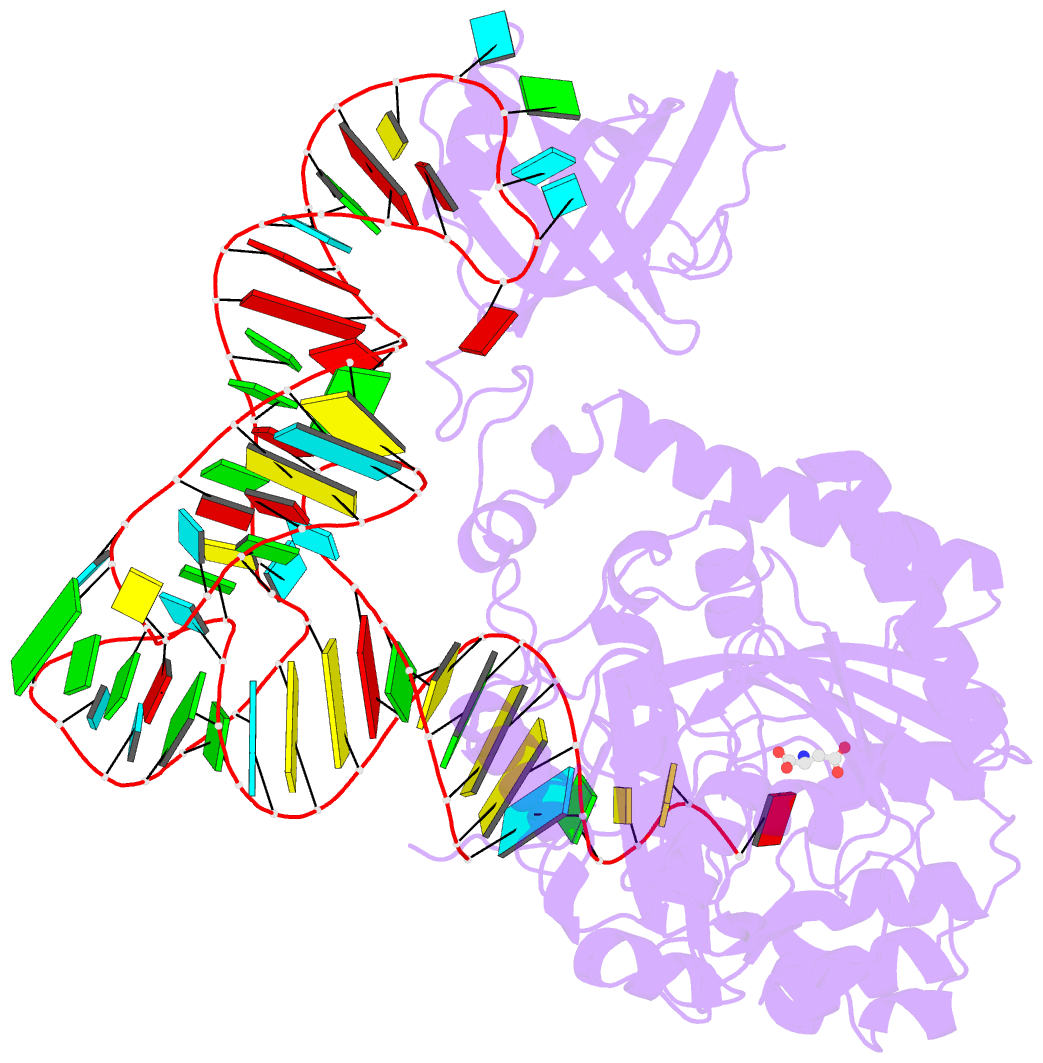
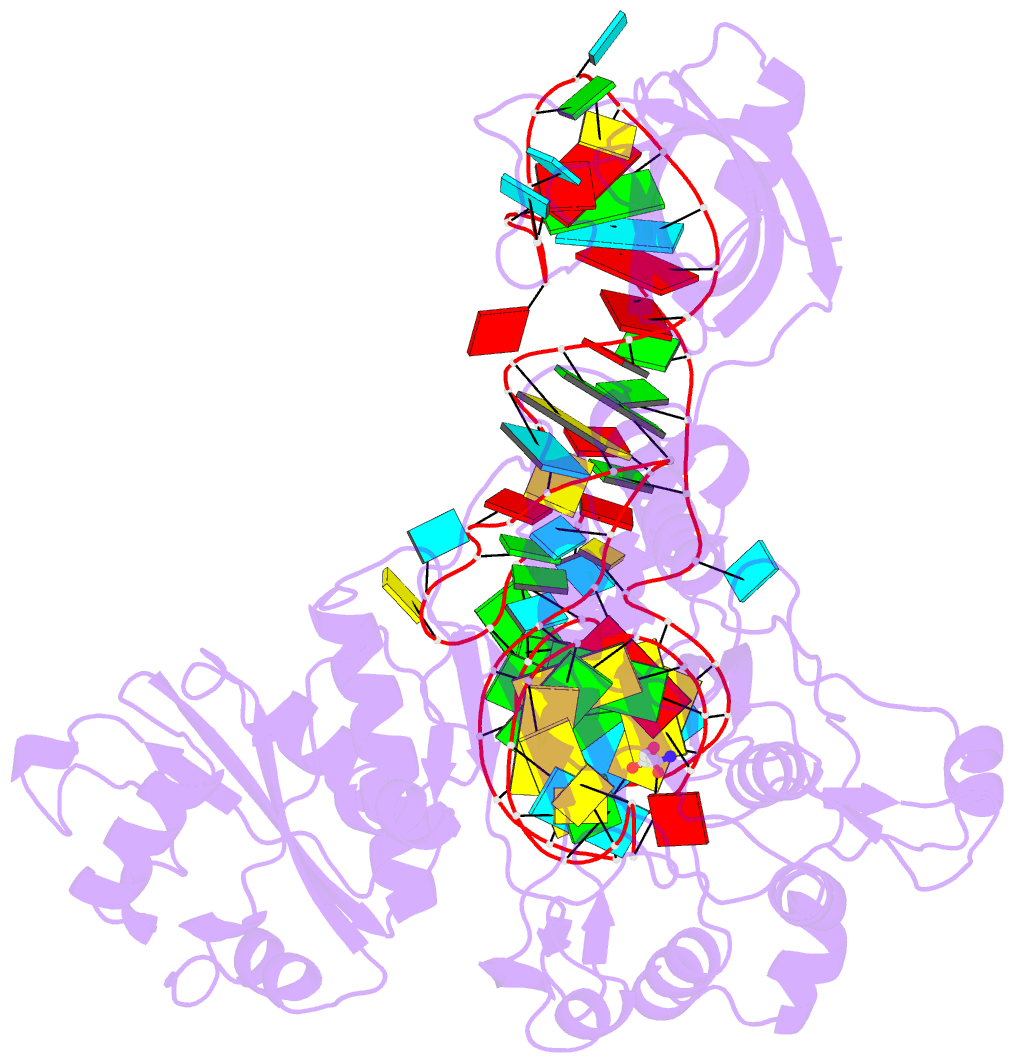
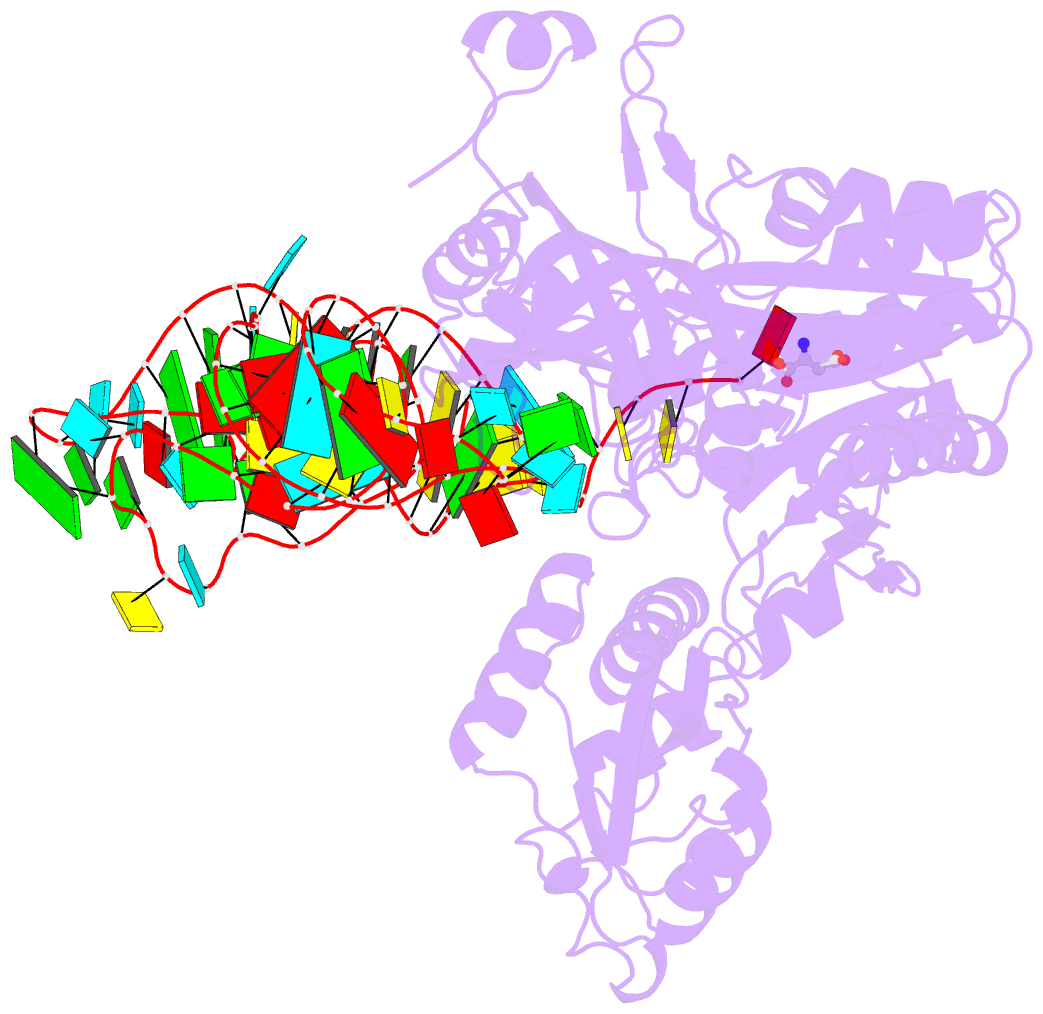
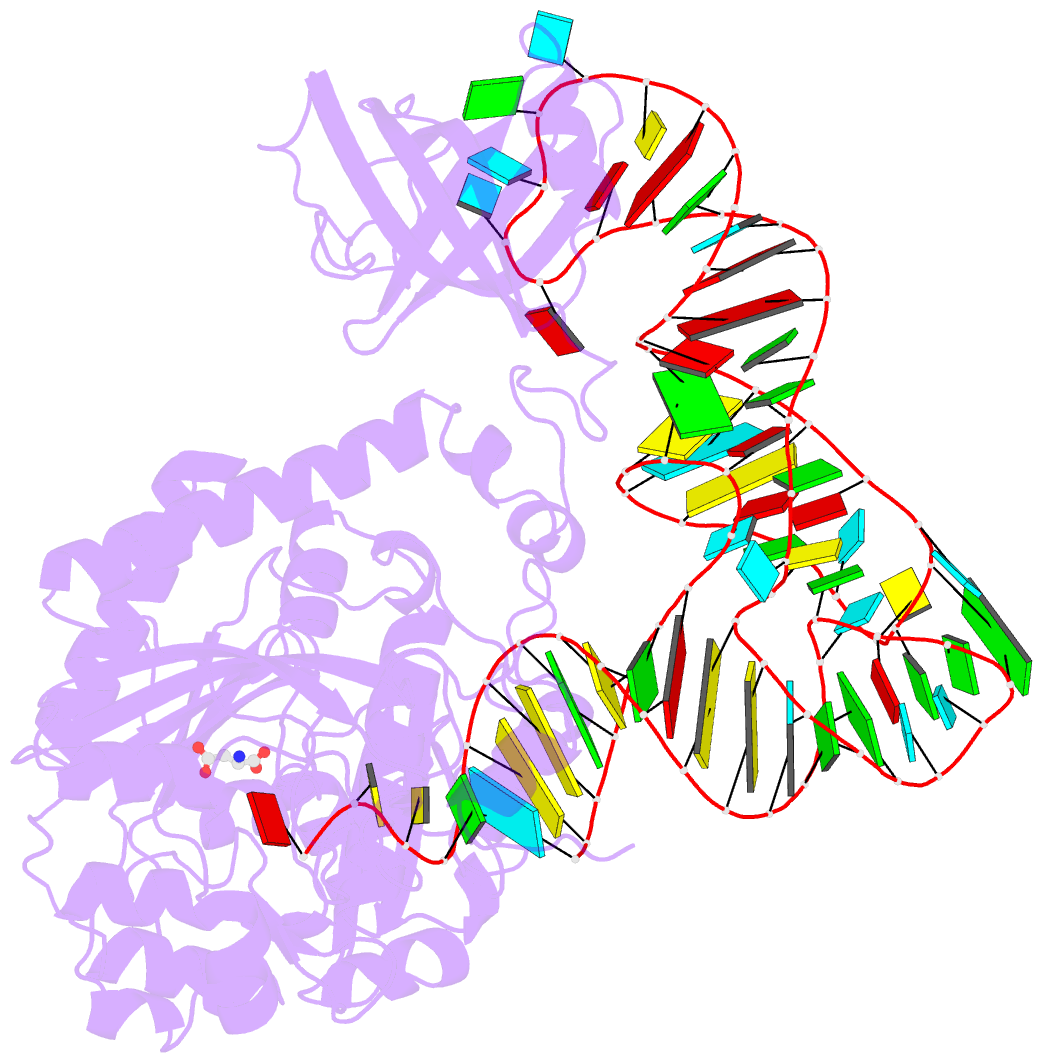
Summary information and primary citation
- PDB-id
- 4wj4; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ligase-RNA
- Method
- X-ray (3.294 Å)
- Summary
- Crystal structure of non-discriminating aspartyl-trna synthetase from pseudomonas aeruginosa complexed with trna(asn) and aspartic acid
- Reference
- Suzuki T, Nakamura A, Kato K, Soll D, Tanaka I, Sheppard K, Yao M (2015): "Structure of the Pseudomonas aeruginosa transamidosome reveals unique aspects of bacterial tRNA-dependent asparagine biosynthesis." Proc.Natl.Acad.Sci.USA, 112, 382-387. doi: 10.1073/pnas.1423314112.
- Abstract
- Many prokaryotes lack a tRNA synthetase to attach asparagine to its cognate tRNA(Asn), and instead synthesize asparagine from tRNA(Asn)-bound aspartate. This conversion involves two enzymes: a nondiscriminating aspartyl-tRNA synthetase (ND-AspRS) that forms Asp-tRNA(Asn), and a heterotrimeric amidotransferase GatCAB that amidates Asp-tRNA(Asn) to form Asn-tRNA(Asn) for use in protein synthesis. ND-AspRS, GatCAB, and tRNA(Asn) may assemble in an ∼400-kDa complex, known as the Asn-transamidosome, which couples the two steps of asparagine biosynthesis in space and time to yield Asn-tRNA(Asn). We report the 3.7-Å resolution crystal structure of the Pseudomonas aeruginosa Asn-transamidosome, which represents the most common machinery for asparagine biosynthesis in bacteria. We show that, in contrast to a previously described archaeal-type transamidosome, a bacteria-specific GAD domain of ND-AspRS provokes a principally new architecture of the complex. Both tRNA(Asn) molecules in the transamidosome simultaneously serve as substrates and scaffolds for the complex assembly. This architecture rationalizes an elevated dynamic and a greater turnover of ND-AspRS within bacterial-type transamidosomes, and possibly may explain a different evolutionary pathway of GatCAB in organisms with bacterial-type vs. archaeal-type Asn-transamidosomes. Importantly, because the two-step pathway for Asn-tRNA(Asn) formation evolutionarily preceded the direct attachment of Asn to tRNA(Asn), our structure also may reflect the mechanism by which asparagine was initially added to the genetic code.