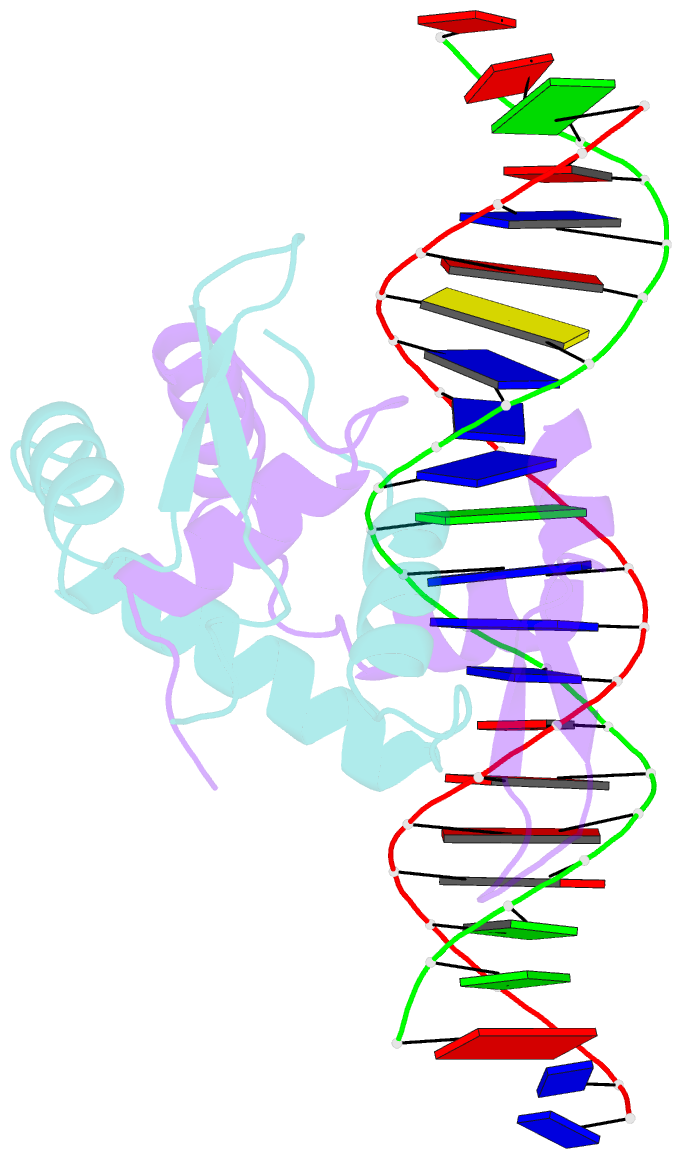
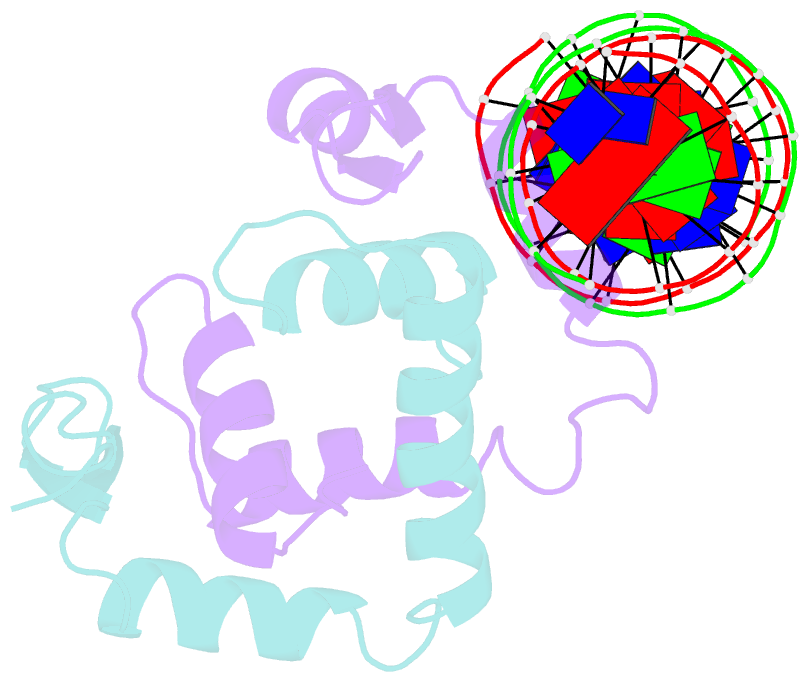
Summary information and primary citation
- PDB-id
- 4wk8; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (3.4 Å)
- Summary
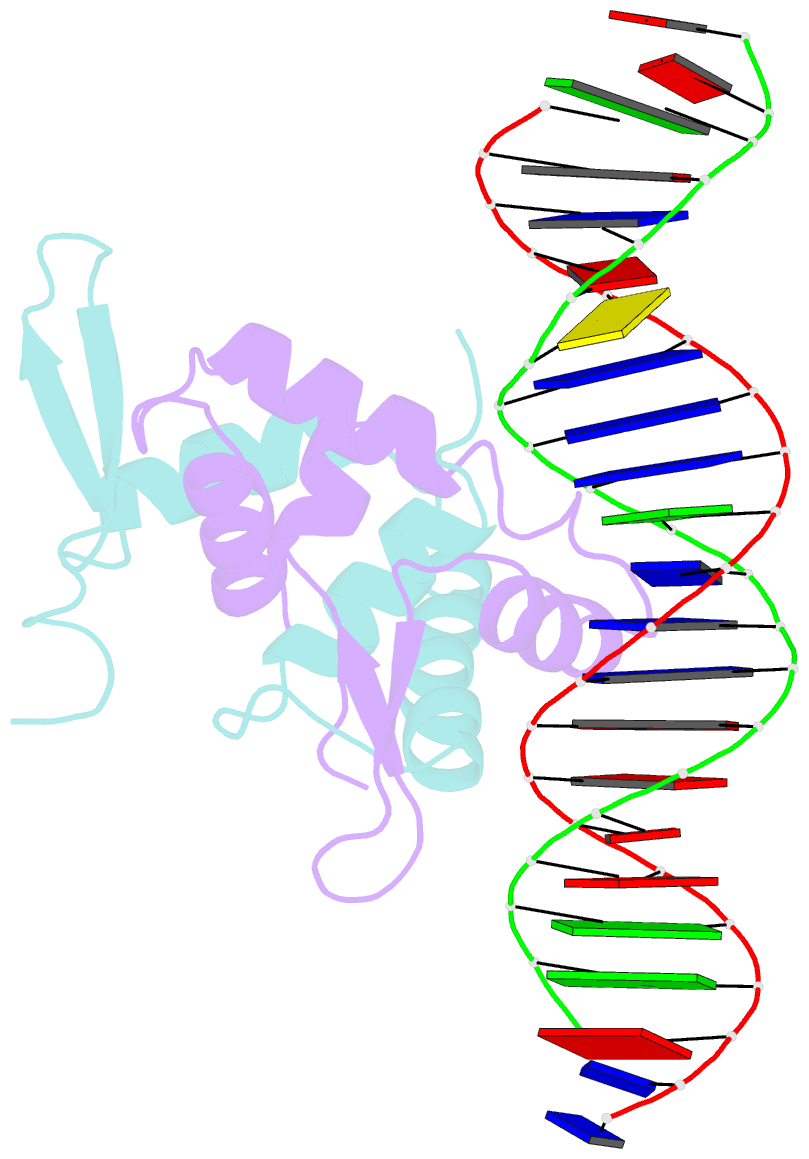
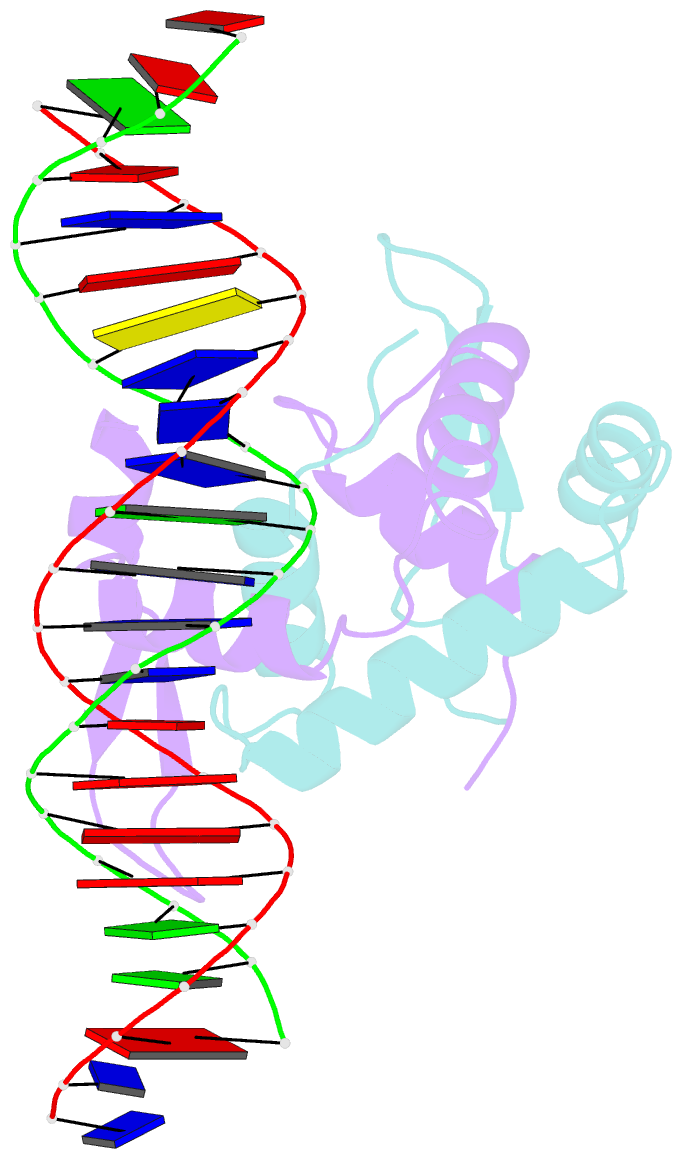
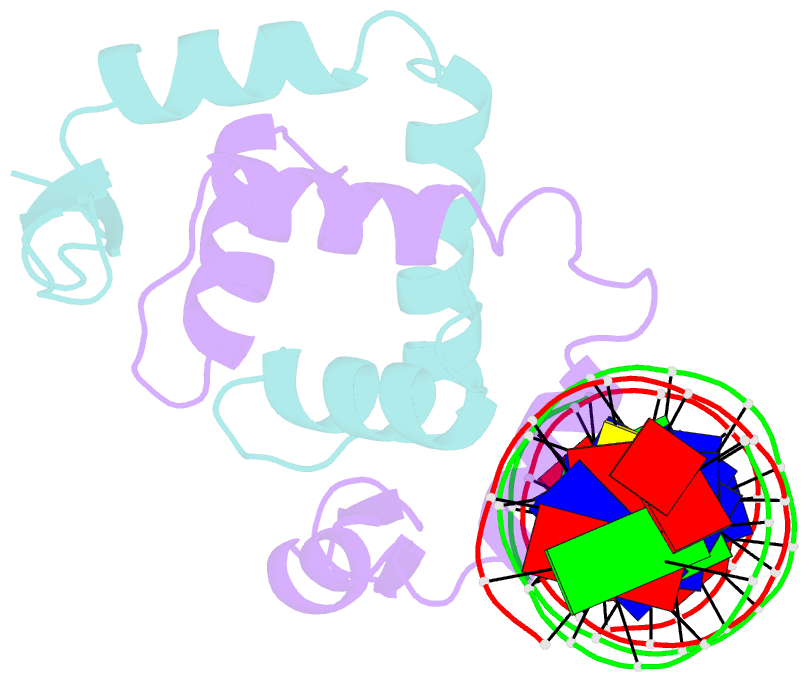
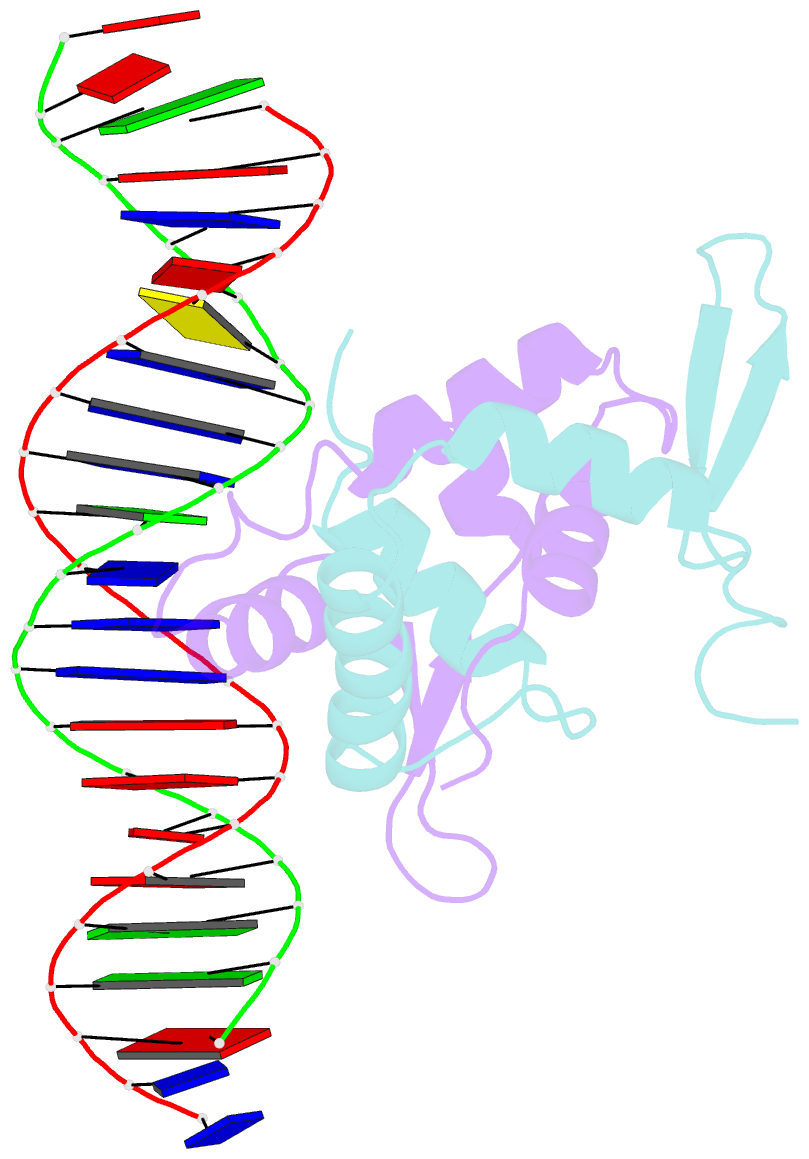
- Foxp3 forms a domain-swapped dimer to bridge DNA
- Reference
- Chen Y, Chen C, Zhang Z, Liu CC, Johnson ME, Espinoza CA, Edsall LE, Ren B, Zhou XJ, Grant SF, Wells AD, Chen L (2015): "DNA binding by FOXP3 domain-swapped dimer suggests mechanisms of long-range chromosomal interactions." Nucleic Acids Res., 43, 1268-1282. doi: 10.1093/nar/gku1373.
- Abstract
- FOXP3 is a lineage-specific transcription factor that is required for regulatory T cell development and function. In this study, we determined the crystal structure of the FOXP3 forkhead domain bound to DNA. The structure reveals that FOXP3 can form a stable domain-swapped dimer to bridge DNA in the absence of cofactors, suggesting that FOXP3 may play a role in long-range gene interactions. To test this hypothesis, we used circular chromosome conformation capture coupled with high throughput sequencing (4C-seq) to analyze FOXP3-dependent genomic contacts around a known FOXP3-bound locus, Ptpn22. Our studies reveal that FOXP3 induces significant changes in the chromatin contacts between the Ptpn22 locus and other Foxp3-regulated genes, reflecting a mechanism by which FOXP3 reorganizes the genome architecture to coordinate the expression of its target genes. Our results suggest that FOXP3 mediates long-range chromatin interactions as part of its mechanisms to regulate specific gene expression in regulatory T cells.