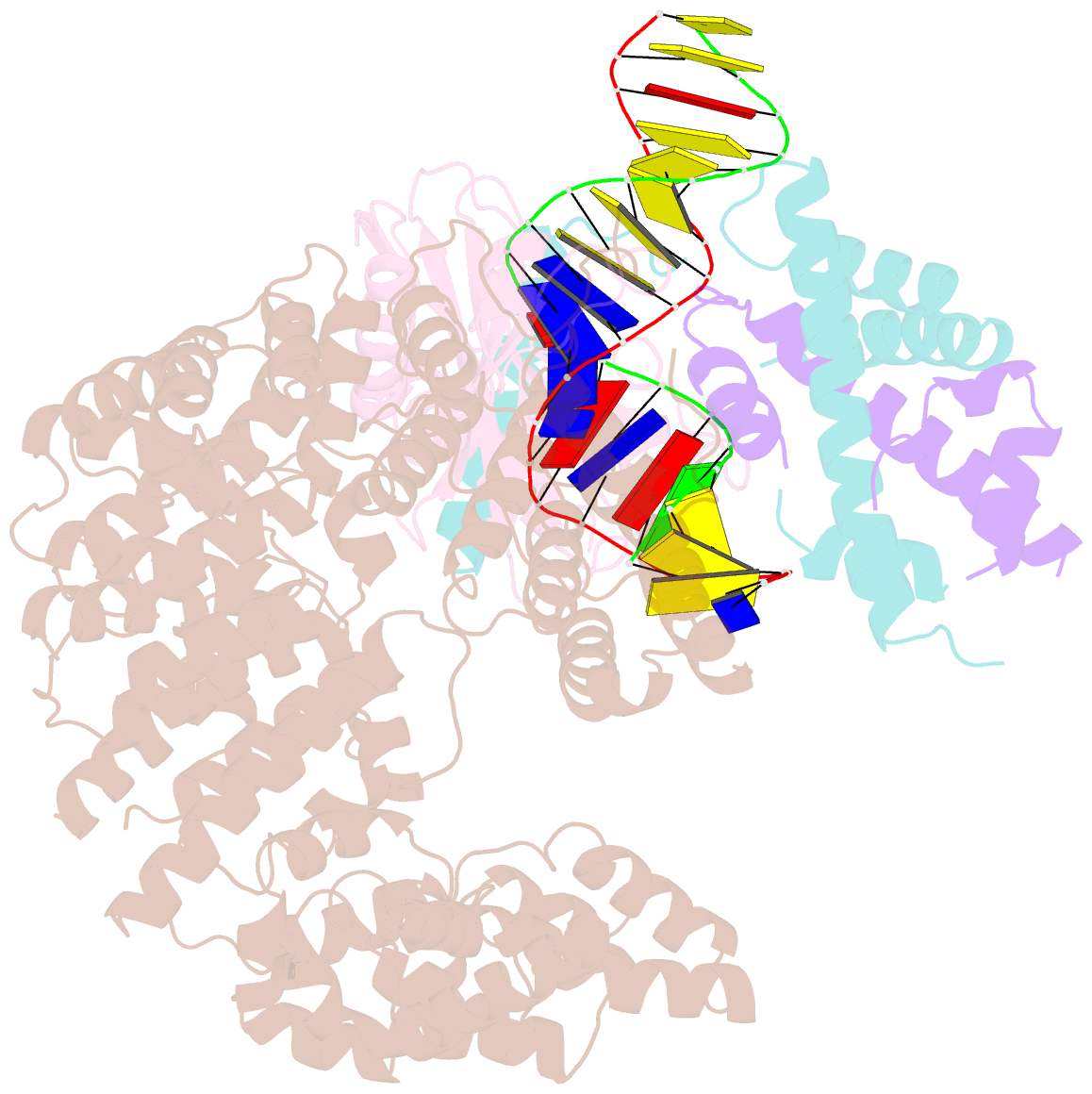
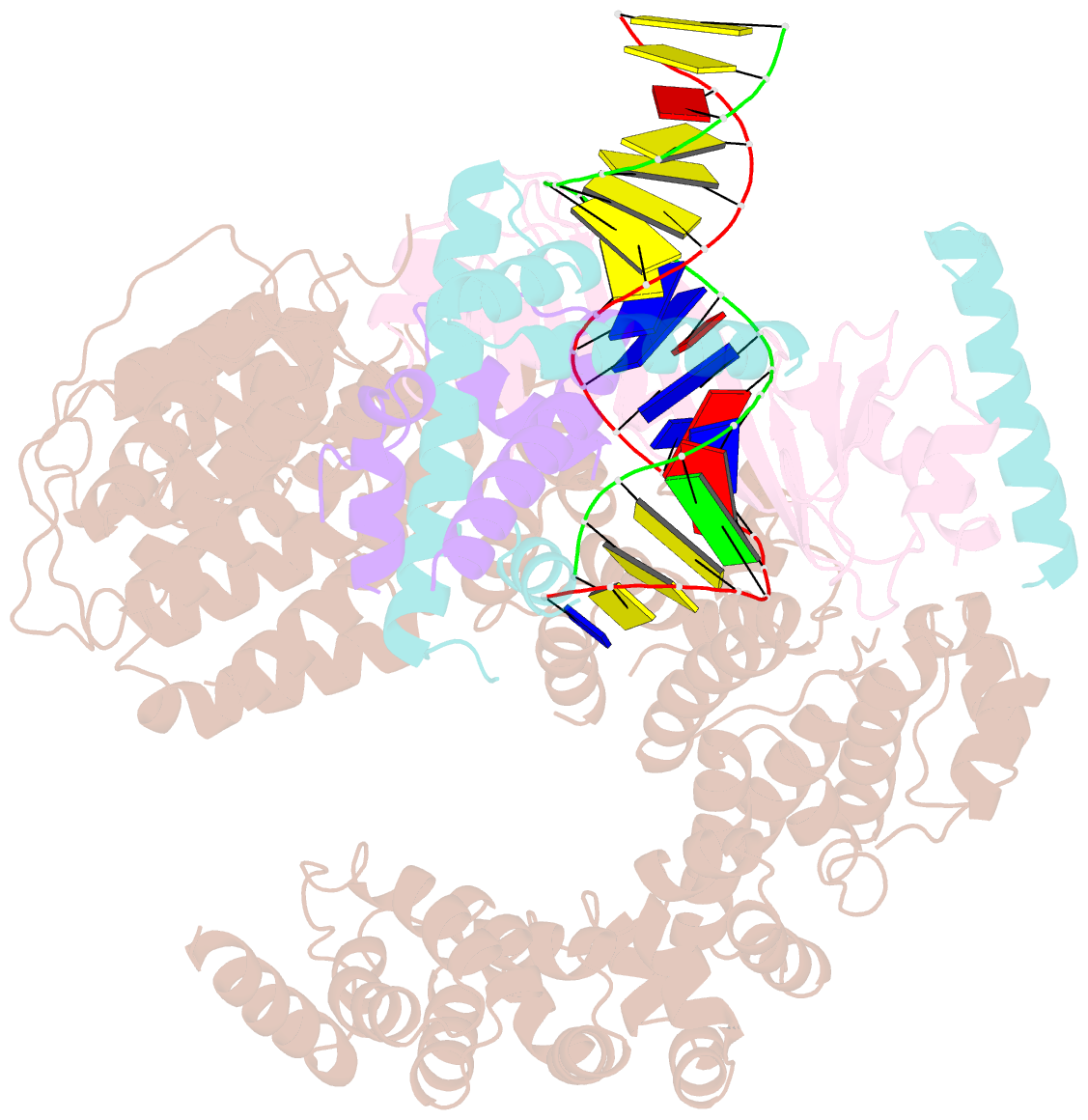
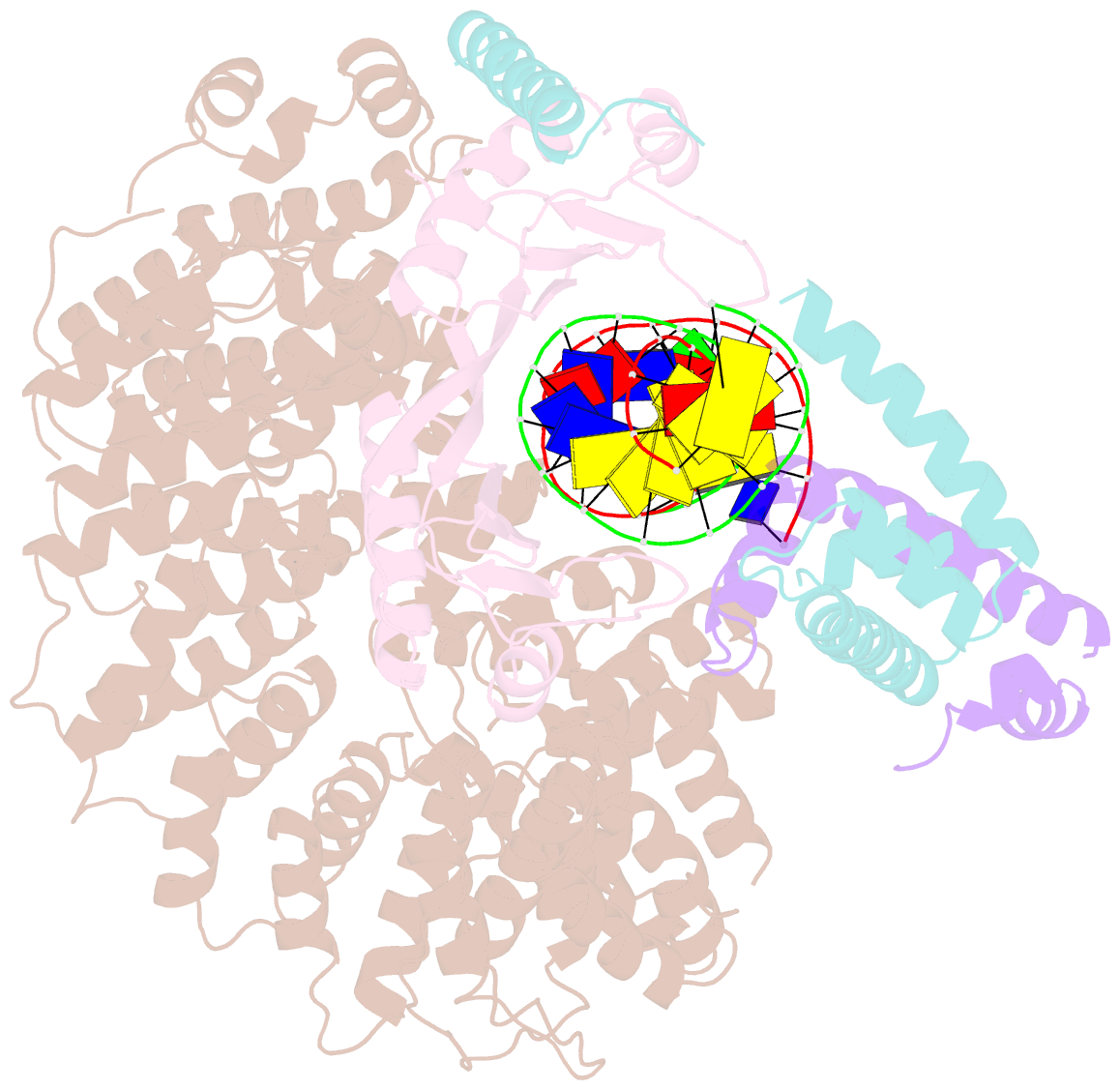
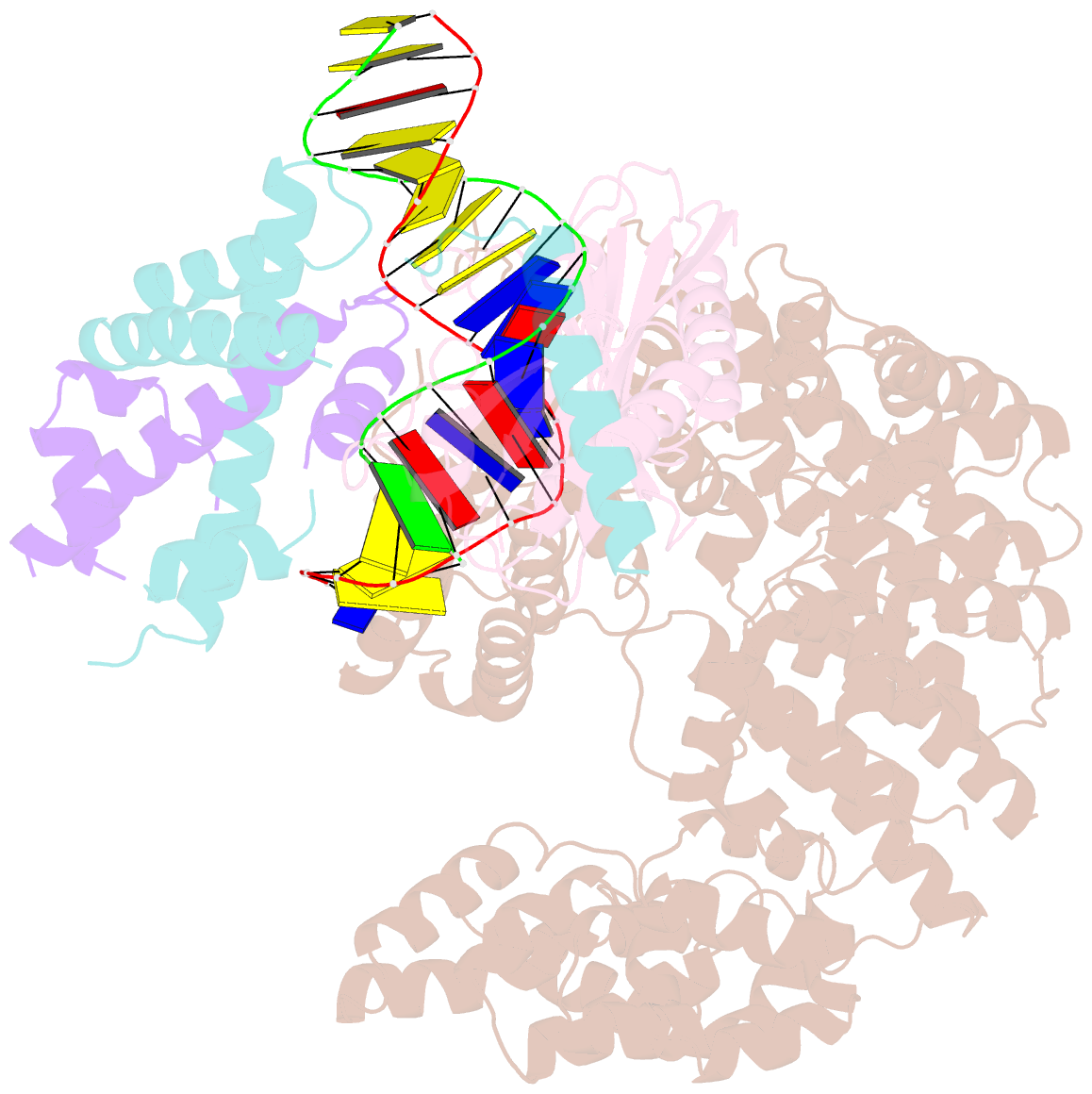
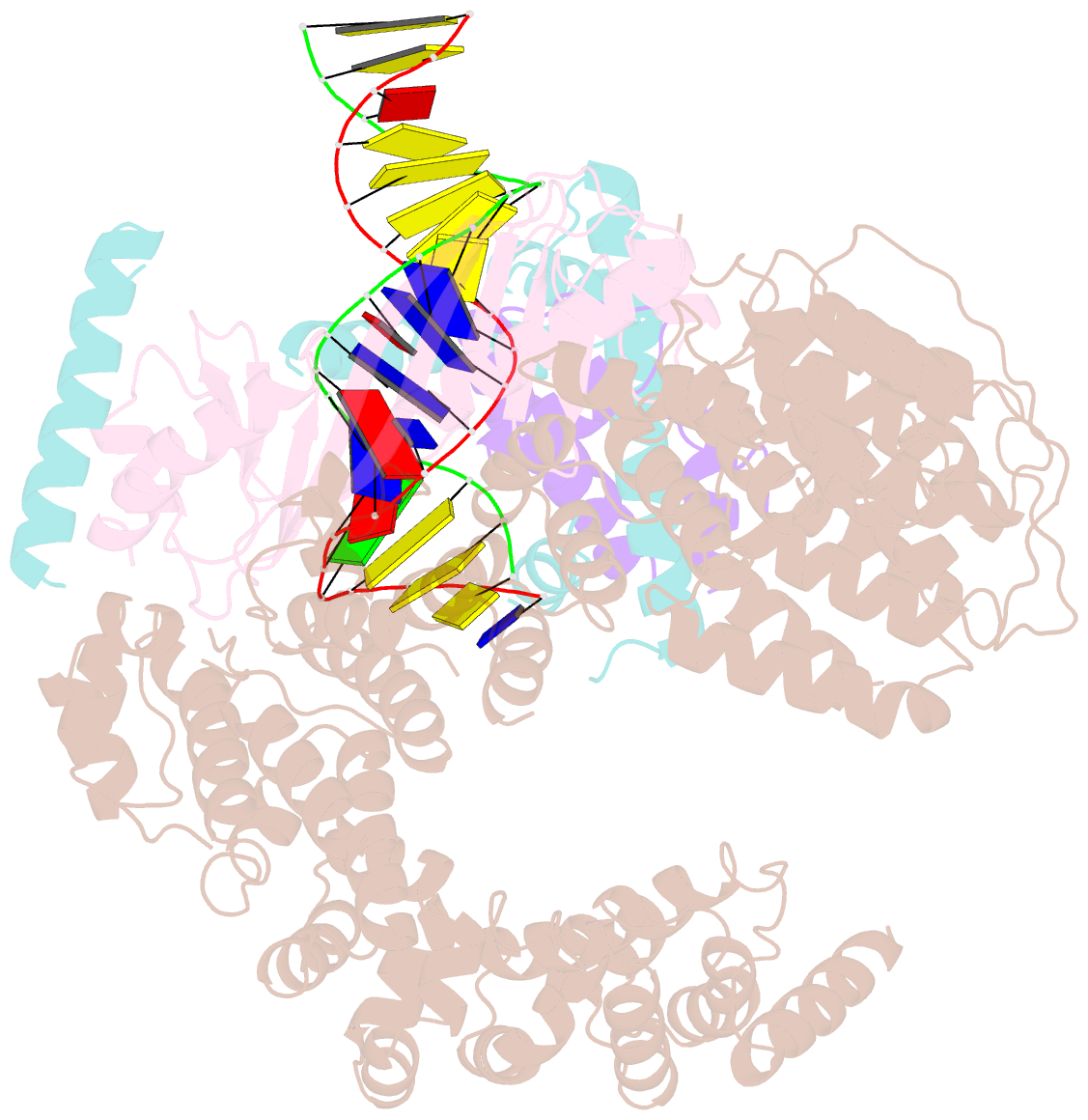
Summary information and primary citation
- PDB-id
- 4wzs; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription
- Method
- X-ray (3.78 Å)
- Summary
- Crystal structure of the mot1 n-terminal domain in complex with tbp and nc2 bound to a promoter DNA fragment
- Reference
- Butryn A, Schuller JM, Stoehr G, Runge-Wollmann P, Forster F, Auble DT, Hopfner KP (2015): "Structural basis for recognition and remodeling of the TBP:DNA:NC2 complex by Mot1." Elife, 4. doi: 10.7554/eLife.07432.
- Abstract
- Swi2/Snf2 ATPases remodel substrates such as nucleosomes and transcription complexes to control a wide range of DNA-associated processes, but detailed structural information on the ATP-dependent remodeling reactions is largely absent. The single subunit remodeler Mot1 (modifier of transcription 1) dissociates TATA box-binding protein (TBP):DNA complexes, offering a useful system to address the structural mechanisms of Swi2/Snf2 ATPases. Here, we report the crystal structure of the N-terminal domain of Mot1 in complex with TBP, DNA, and the transcription regulator negative cofactor 2 (NC2). Our data show that Mot1 reduces DNA:NC2 interactions and unbends DNA as compared to the TBP:DNA:NC2 state, suggesting that Mot1 primes TBP:NC2 displacement in an ATP-independent manner. Electron microscopy and cross-linking data suggest that the Swi2/Snf2 domain of Mot1 associates with the upstream DNA and the histone fold of NC2, thereby revealing parallels to some nucleosome remodelers. This study provides a structural framework for how a Swi2/Snf2 ATPase interacts with its substrate DNA:protein complex.