Summary information and primary citation
- PDB-id
- 4wzw; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein, RNA binding protein-DNA
- Method
- X-ray (2.95 Å)
- Summary
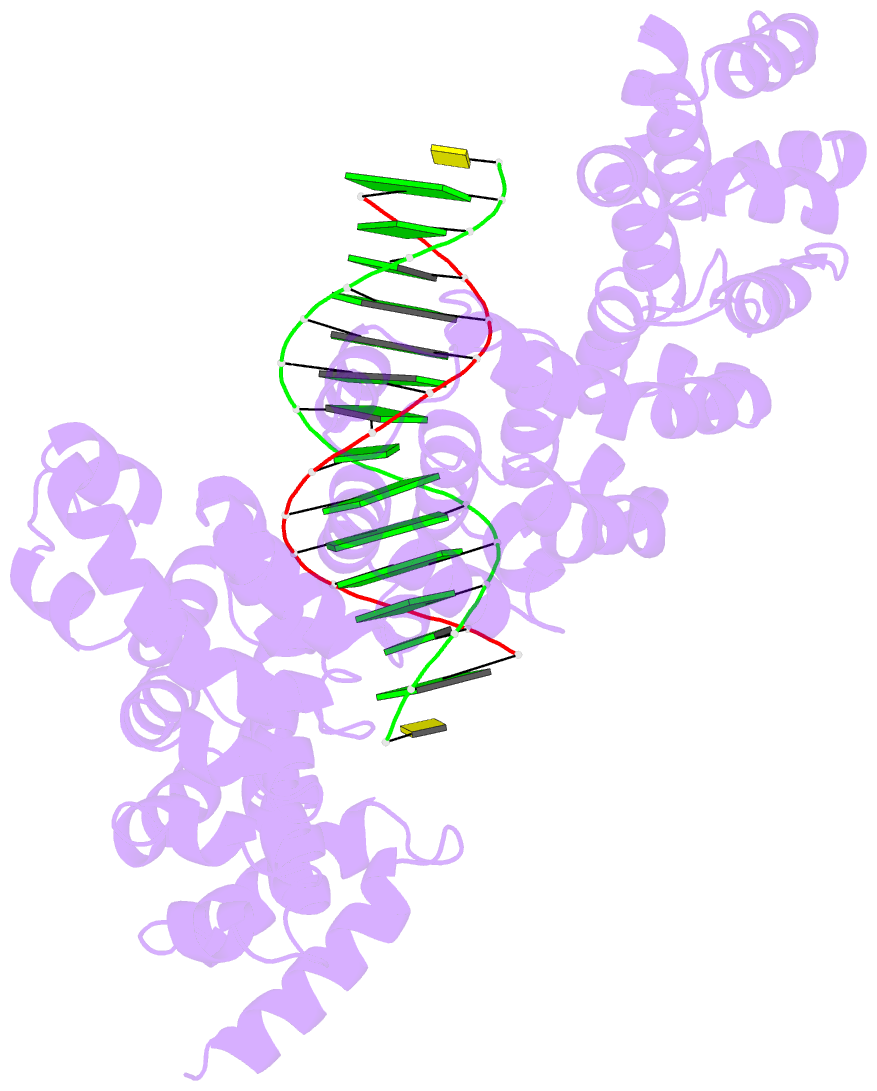
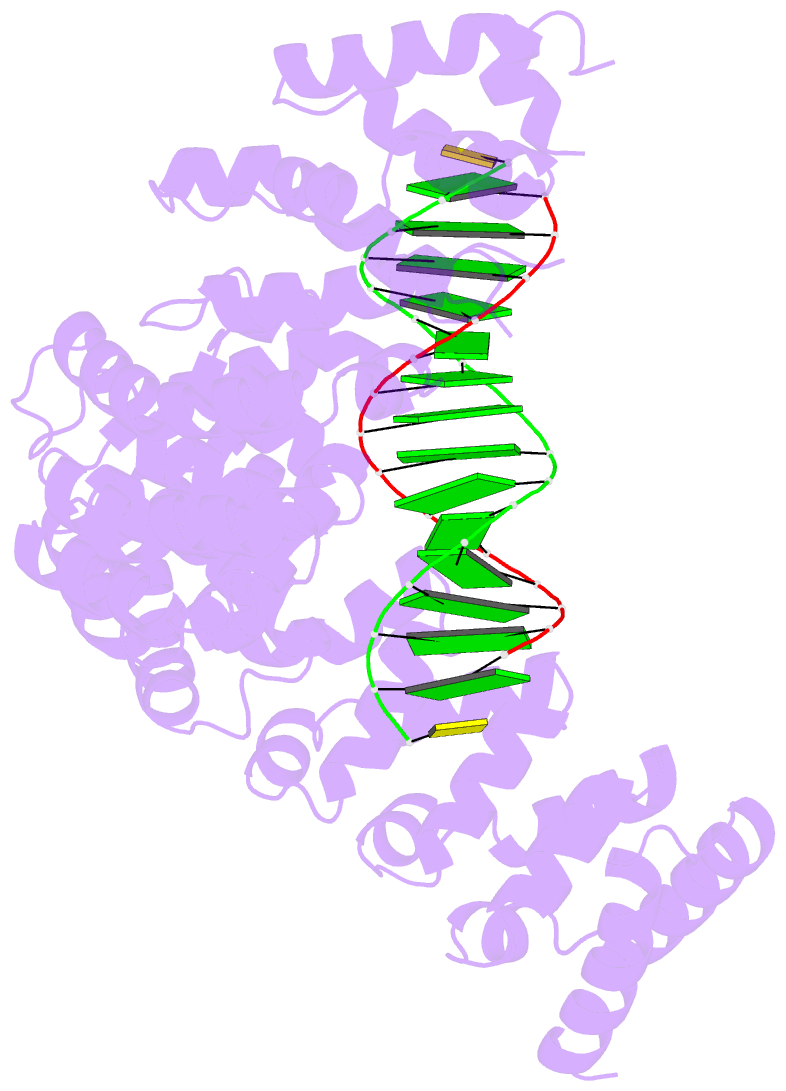
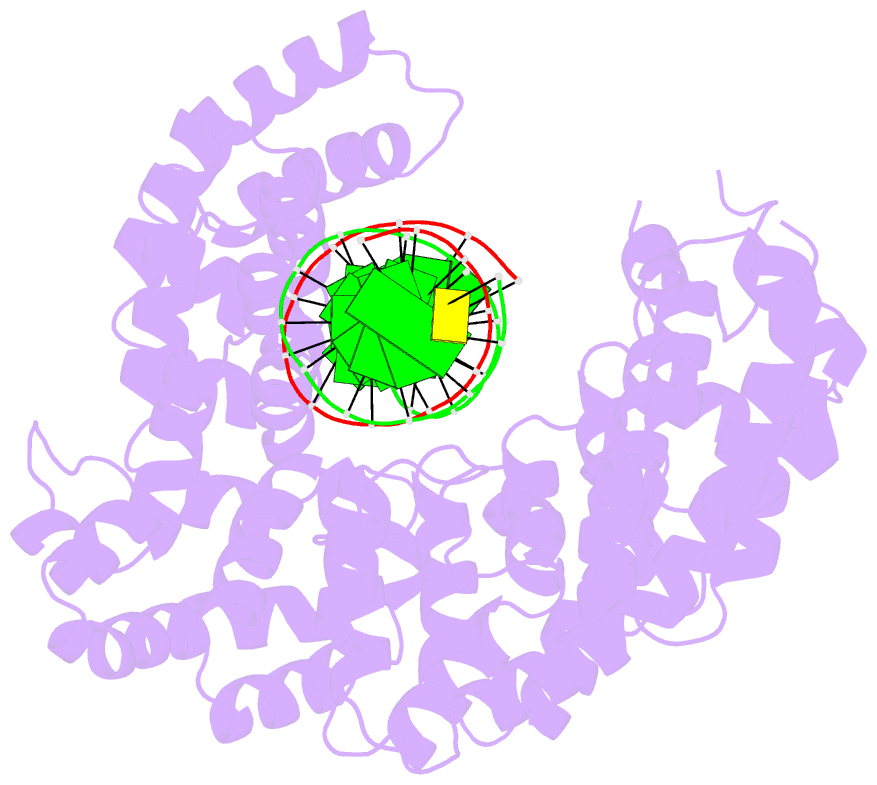
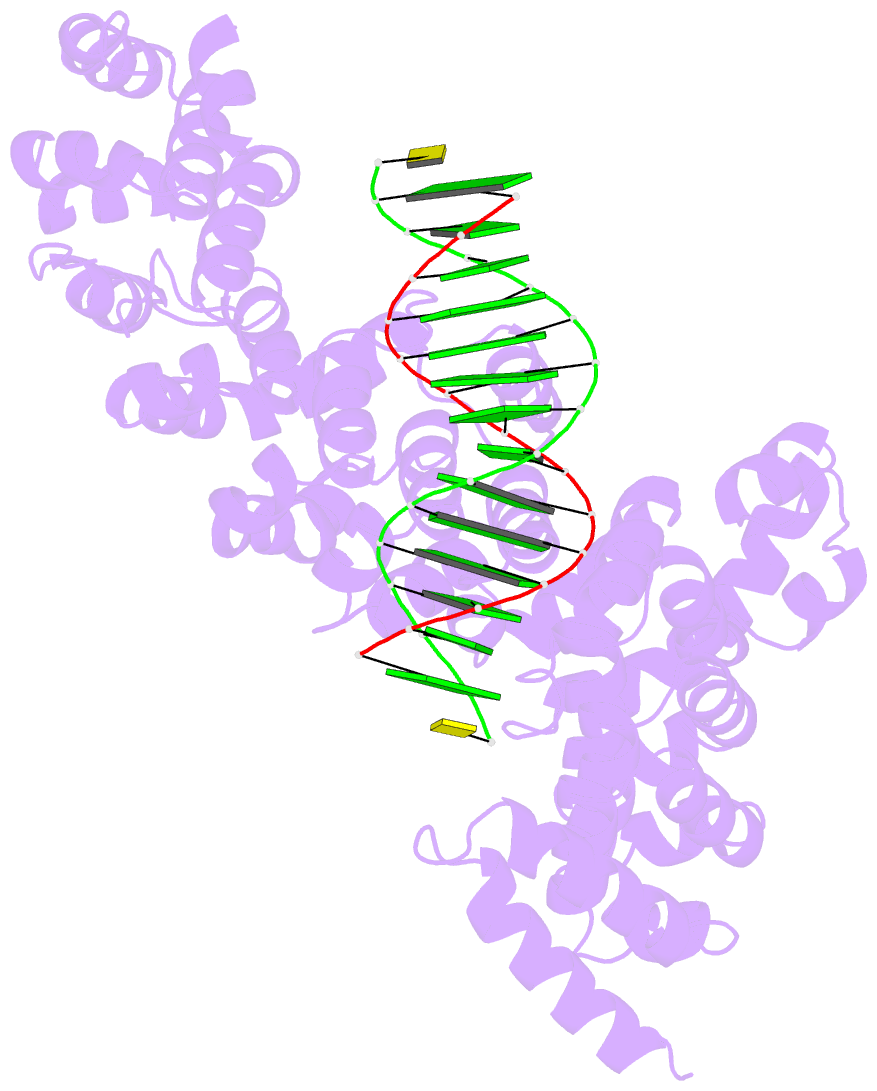
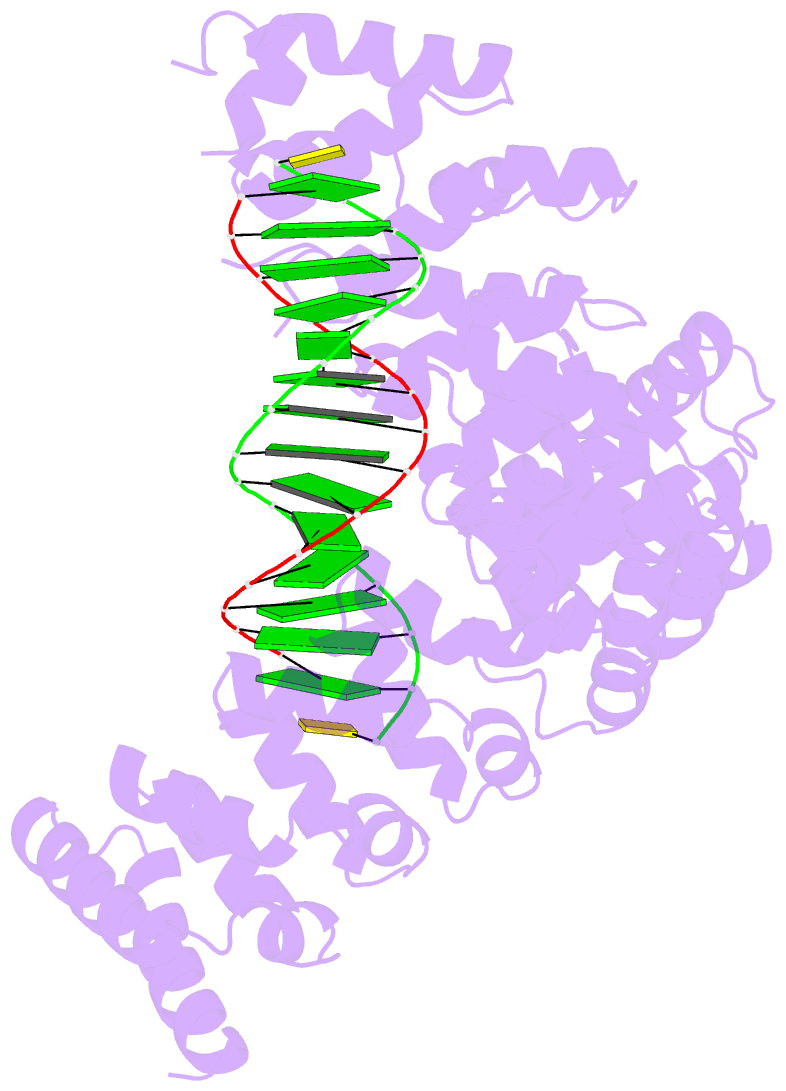
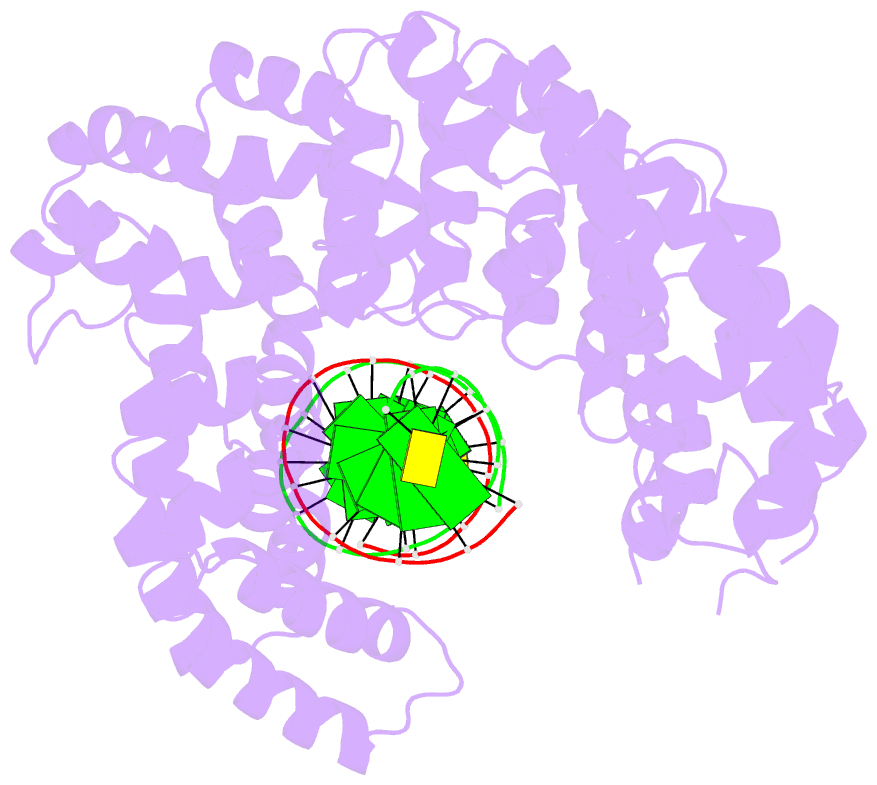
- Crystal structure of human puf-a in complex with DNA
- Reference
- Qiu C, McCann KL, Wine RN, Baserga SJ, Hall TM (2014): "A divergent Pumilio repeat protein family for pre-rRNA processing and mRNA localization." Proc.Natl.Acad.Sci.USA, 111, 18554-18559. doi: 10.1073/pnas.1407634112.
- Abstract
- Pumilio/feminization of XX and XO animals (fem)-3 mRNA-binding factor (PUF) proteins bind sequence specifically to mRNA targets using a single-stranded RNA-binding domain comprising eight Pumilio (PUM) repeats. PUM repeats have now been identified in proteins that function in pre-rRNA processing, including human Puf-A and yeast Puf6. This is a role not previously ascribed to PUF proteins. Here we present crystal structures of human Puf-A that reveal a class of nucleic acid-binding proteins with 11 PUM repeats arranged in an "L"-like shape. In contrast to classical PUF proteins, Puf-A forms sequence-independent interactions with DNA or RNA, mediated by conserved basic residues. We demonstrate that equivalent basic residues in yeast Puf6 are important for RNA binding, pre-rRNA processing, and mRNA localization. Thus, PUM repeats can be assembled into alternative folds that bind to structured nucleic acids in addition to forming canonical eight-repeat crescent-shaped RNA-binding domains found in classical PUF proteins.