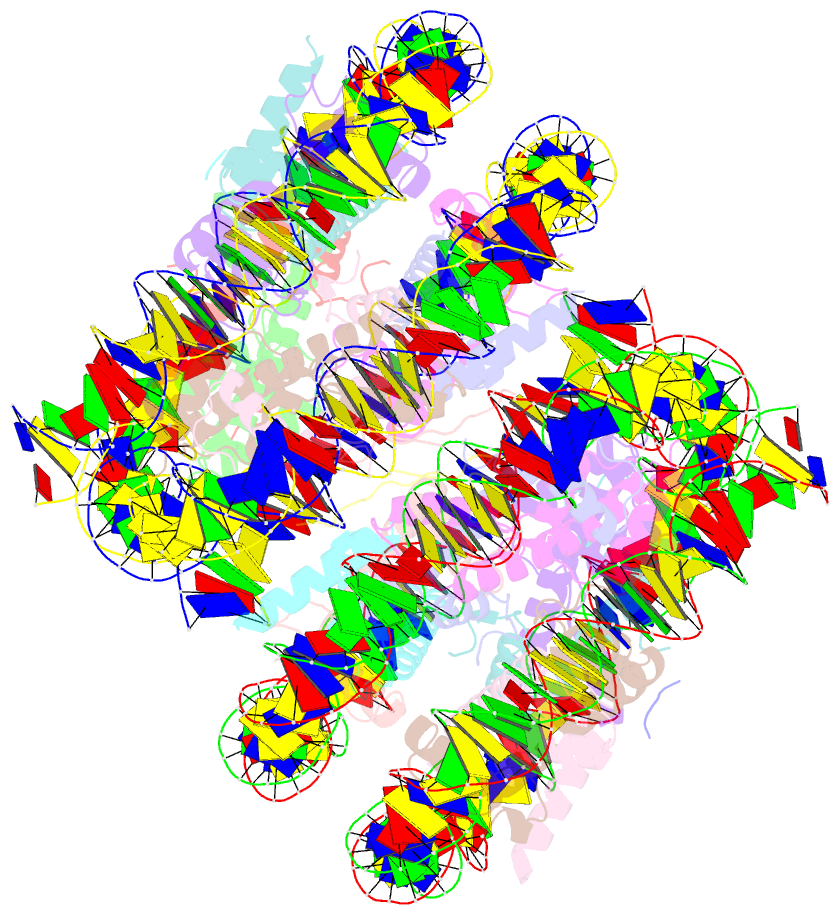
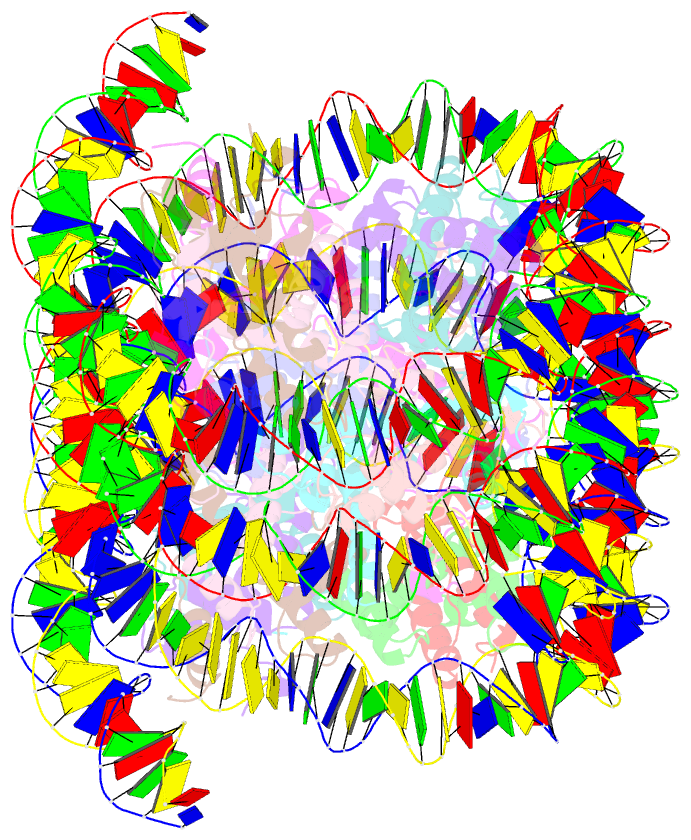
Summary information and primary citation
- PDB-id
- 4x23; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- structural protein-DNA
- Method
- X-ray (3.5 Å)
- Summary
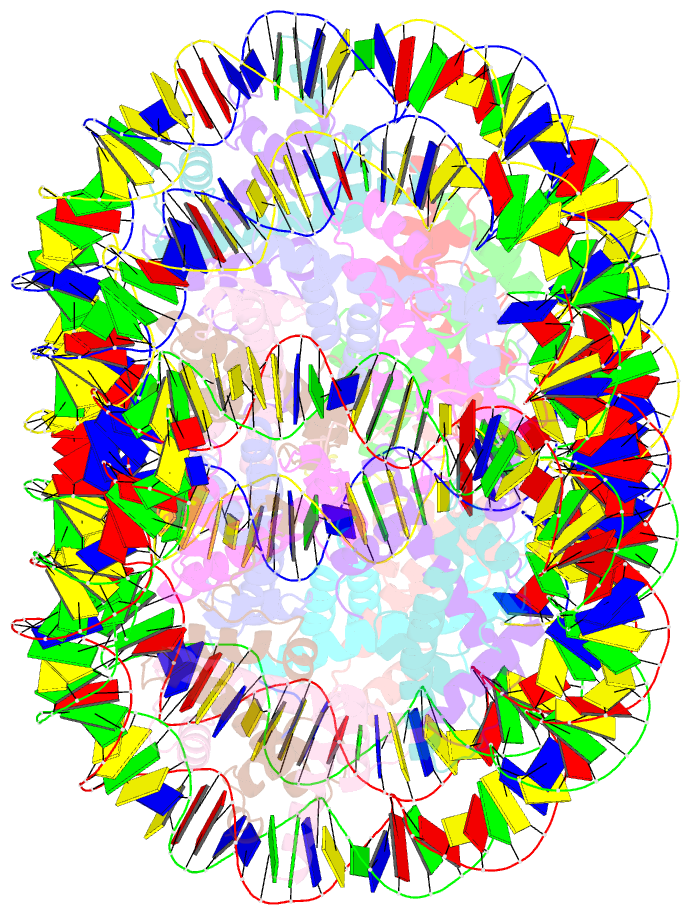
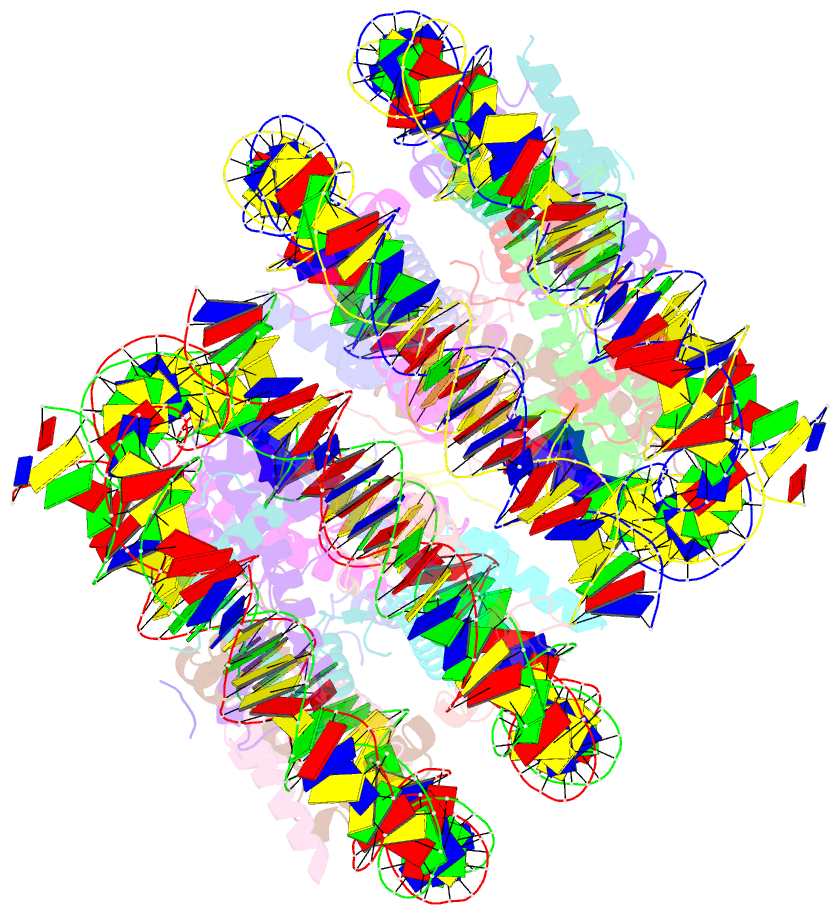
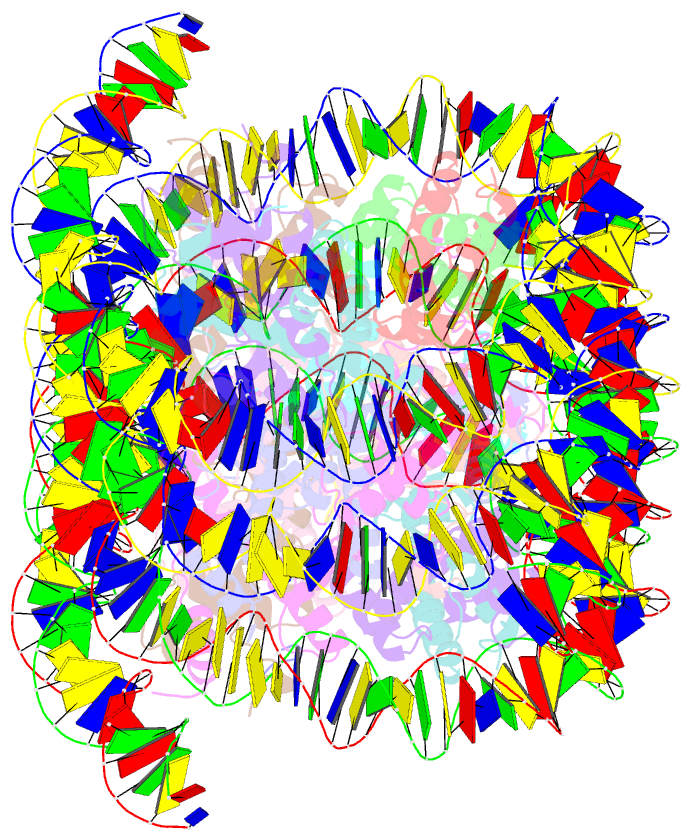
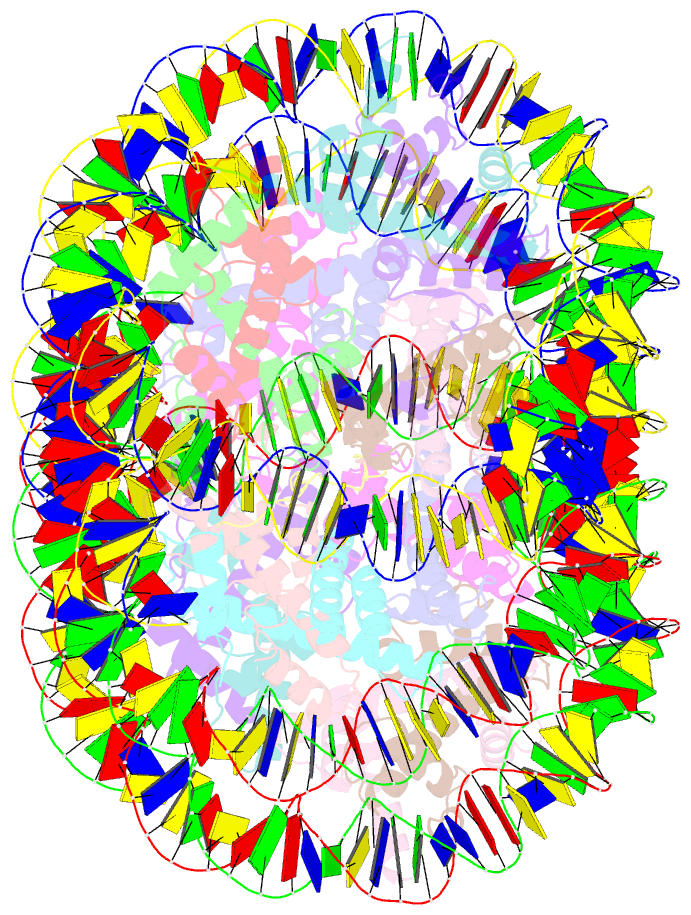
- Crystal structure of cenp-c in complex with the nucleosome core particle
- Reference
- Kato H, Jiang JS, Zhou BR, Rozendaal M, Feng H, Ghirlando R, Xiao TS, Straight AF, Bai Y (2013): "A conserved mechanism for centromeric nucleosome recognition by centromere protein CENP-C." Science, 340, 1110-1113. doi: 10.1126/science.1235532.
- Abstract
- Chromosome segregation during mitosis requires assembly of the kinetochore complex at the centromere. Kinetochore assembly depends on specific recognition of the histone variant CENP-A in the centromeric nucleosome by centromere protein C (CENP-C). We have defined the determinants of this recognition mechanism and discovered that CENP-C binds a hydrophobic region in the CENP-A tail and docks onto the acidic patch of histone H2A and H2B. We further found that the more broadly conserved CENP-C motif uses the same mechanism for CENP-A nucleosome recognition. Our findings reveal a conserved mechanism for protein recruitment to centromeres and a histone recognition mode whereby a disordered peptide binds the histone tail through hydrophobic interactions facilitated by nucleosome docking.