Summary information and primary citation
- PDB-id
- 4yd2; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (2.471 Å)
- Summary
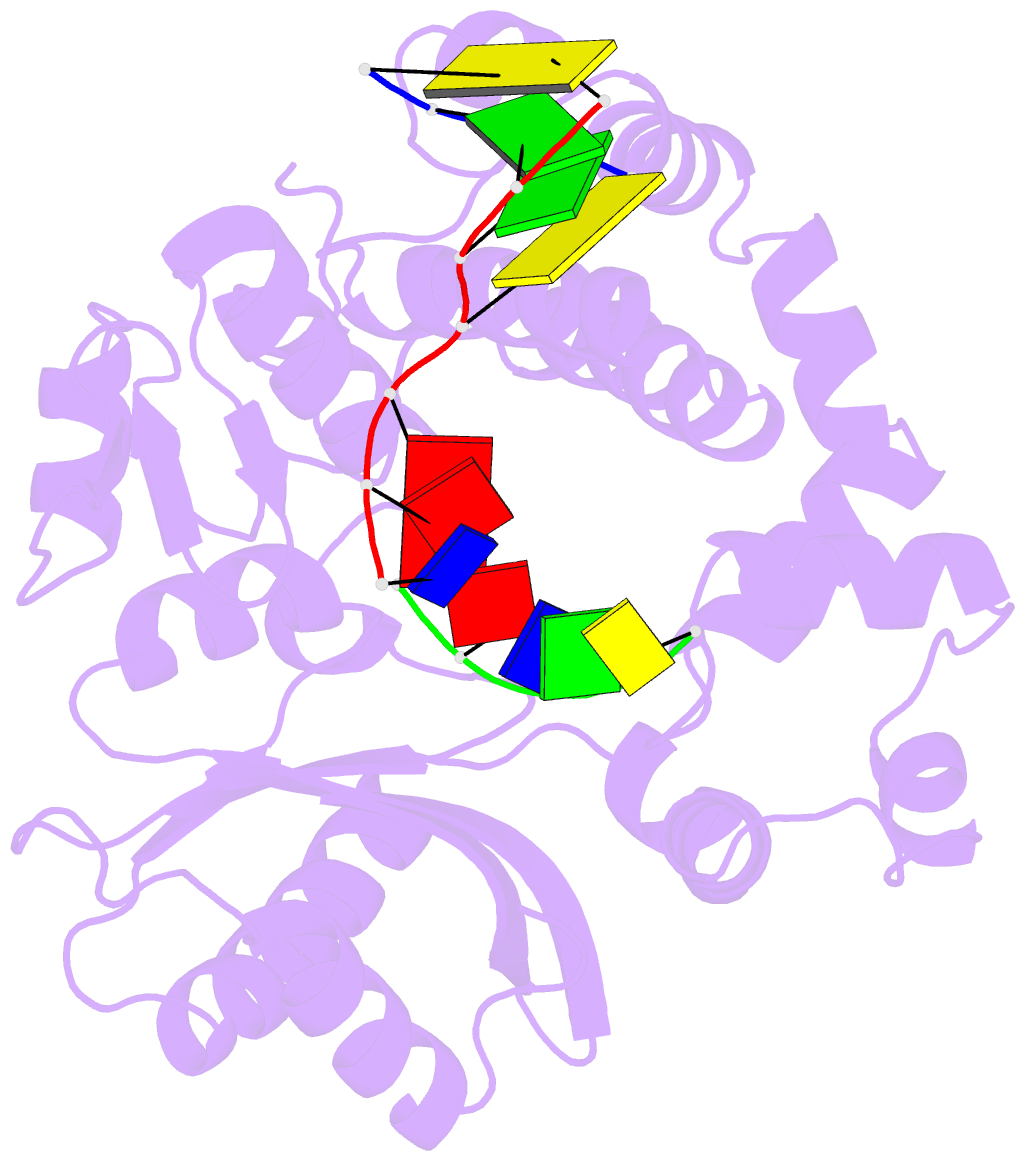
- Nicked complex of human DNA polymerase mu with 2-nt gapped DNA substrate
- Reference
- Moon AF, Gosavi RA, Kunkel TA, Pedersen LC, Bebenek K (2015): "Creative template-dependent synthesis by human polymerase mu." Proc.Natl.Acad.Sci.USA, 112, E4530-E4536. doi: 10.1073/pnas.1505798112.
- Abstract
- Among the many proteins used to repair DNA double-strand breaks by nonhomologous end joining (NHEJ) are two related family X DNA polymerases, Pol λ and Pol µ. Which of these two polymerases is preferentially used for filling DNA gaps during NHEJ partly depends on sequence complementarity at the break, with Pol λ and Pol µ repairing complementary and noncomplementary ends, respectively. To better understand these substrate preferences, we present crystal structures of Pol µ on a 2-nt gapped DNA substrate, representing three steps of the catalytic cycle. In striking contrast to Pol λ, Pol µ "skips" the first available template nucleotide, instead using the template base at the 5' end of the gap to direct nucleotide binding and incorporation. This remarkable divergence from canonical 3'-end gap filling is consistent with data on end-joining substrate specificity in cells, and provides insights into polymerase substrate choices during NHEJ.