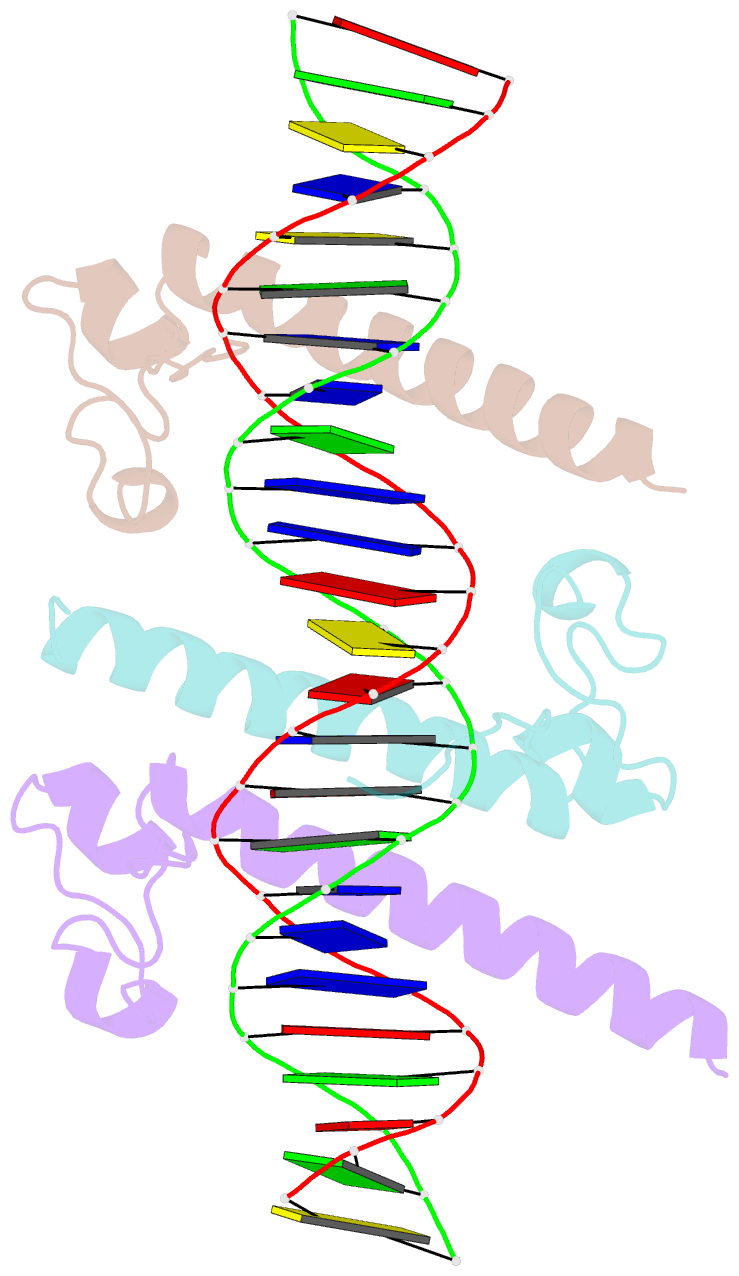
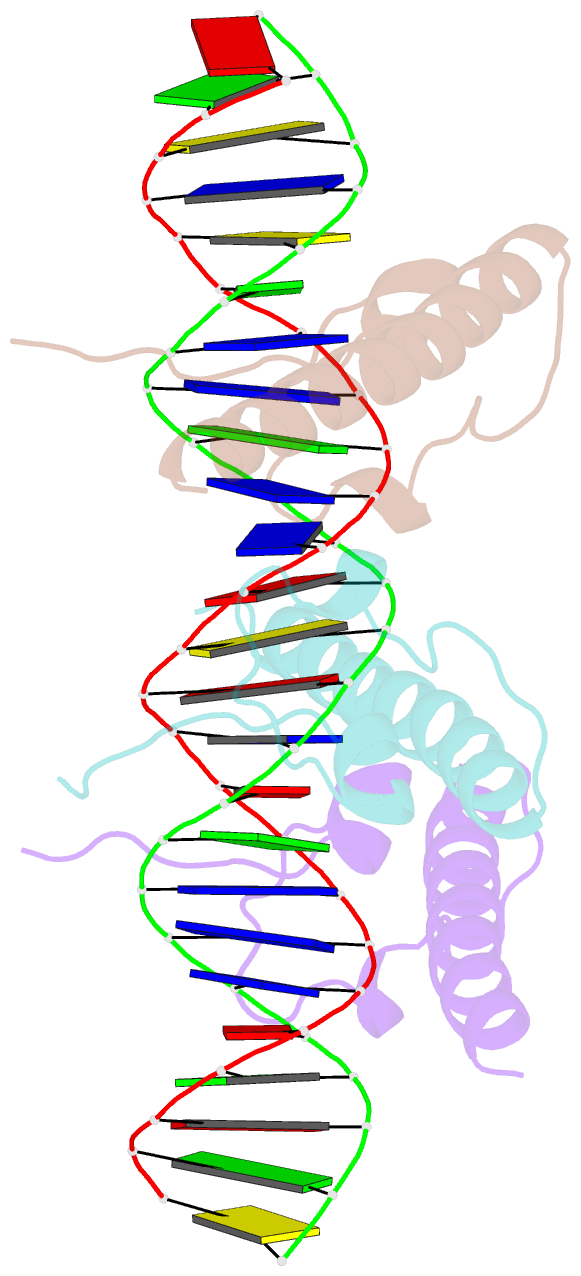
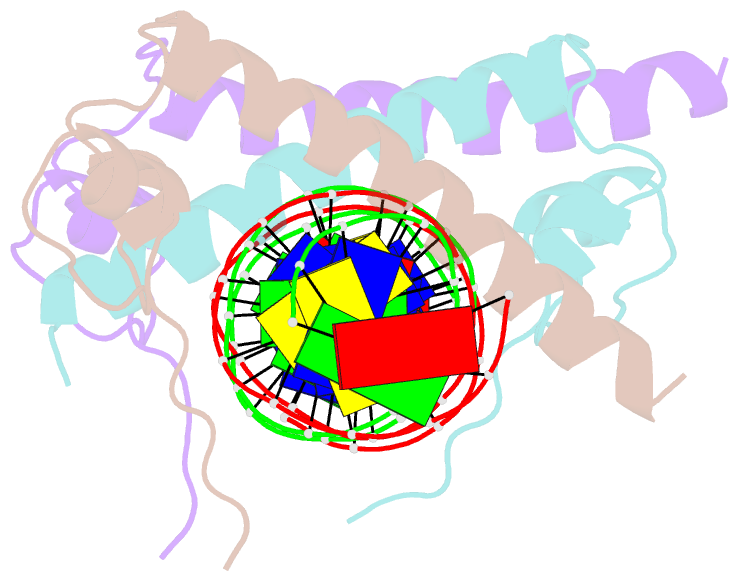
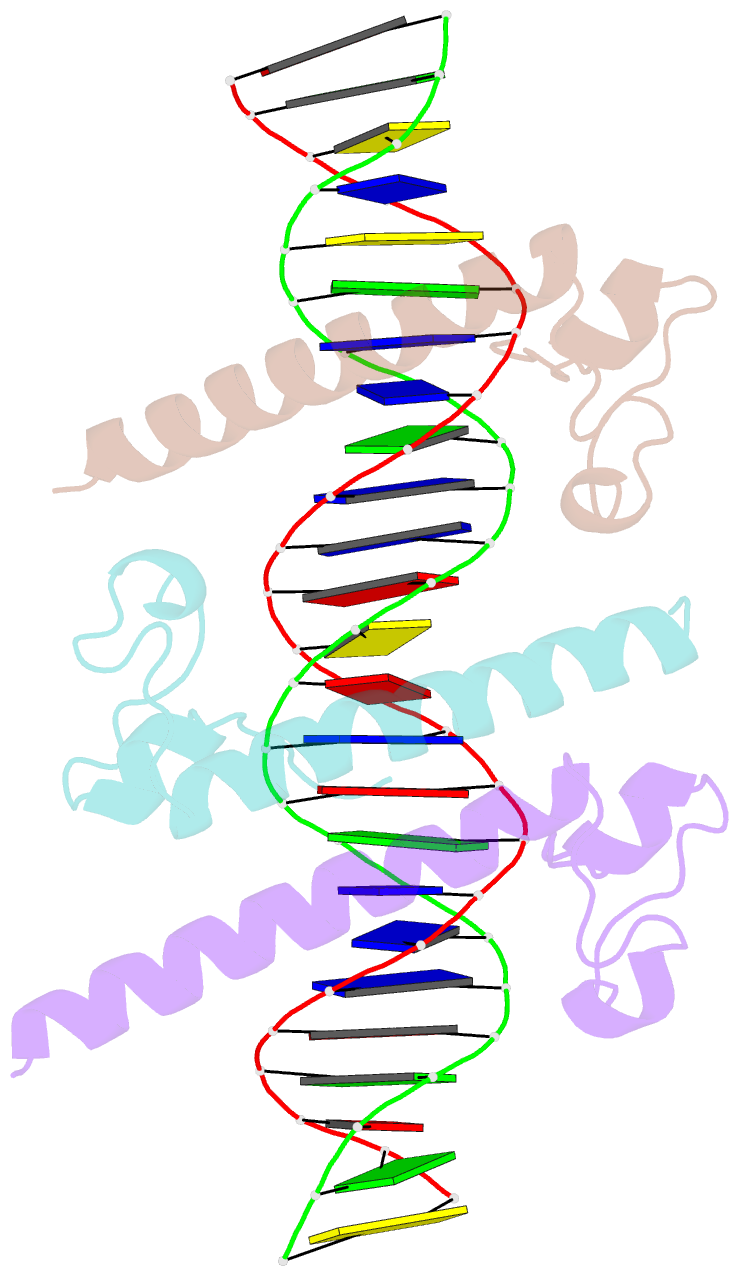
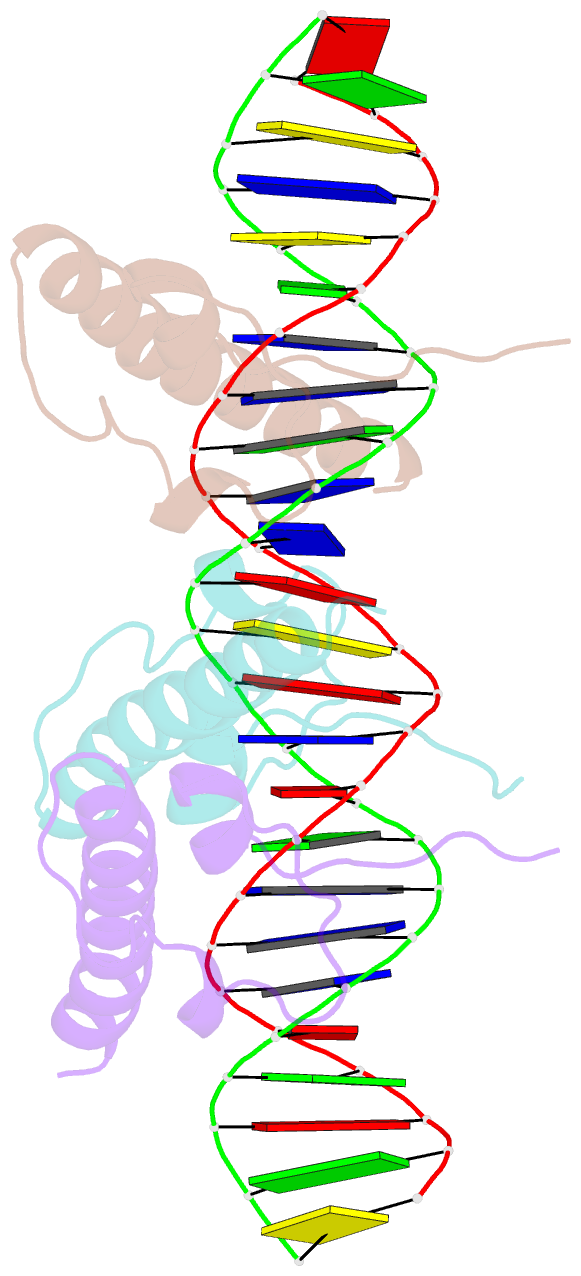
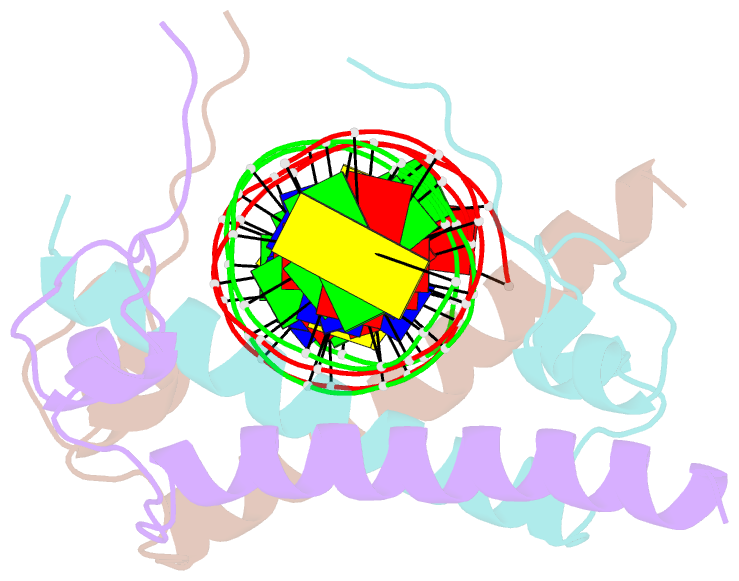
Summary information and primary citation
- PDB-id
- 4yj0; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription
- Method
- X-ray (3.814 Å)
- Summary
- Crystal structure of the dm domain of human dmrt1 bound to 25mer target DNA
- Reference
- Murphy MW, Lee JK, Rojo S, Gearhart MD, Kurahashi K, Banerjee S, Loeuille GA, Bashamboo A, McElreavey K, Zarkower D, Aihara H, Bardwell VJ (2015): "An ancient protein-DNA interaction underlying metazoan sex determination." Nat.Struct.Mol.Biol., 22, 442-451. doi: 10.1038/nsmb.3032.
- Abstract
- DMRT transcription factors are deeply conserved regulators of metazoan sexual development. They share the DM DNA-binding domain, a unique intertwined double zinc-binding module followed by a C-terminal recognition helix, which binds a pseudopalindromic target DNA. Here we show that DMRT proteins use a unique binding interaction, inserting two adjacent antiparallel recognition helices into a widened DNA major groove to make base-specific contacts. Versatility in how specific base contacts are made allows human DMRT1 to use multiple DNA binding modes (tetramer, trimer and dimer). Chromatin immunoprecipitation with exonuclease treatment (ChIP-exo) indicates that multiple DNA binding modes also are used in vivo. We show that mutations affecting residues crucial for DNA recognition are associated with an intersex phenotype in flies and with male-to-female sex reversal in humans. Our results illuminate an ancient molecular interaction underlying much of metazoan sexual development.