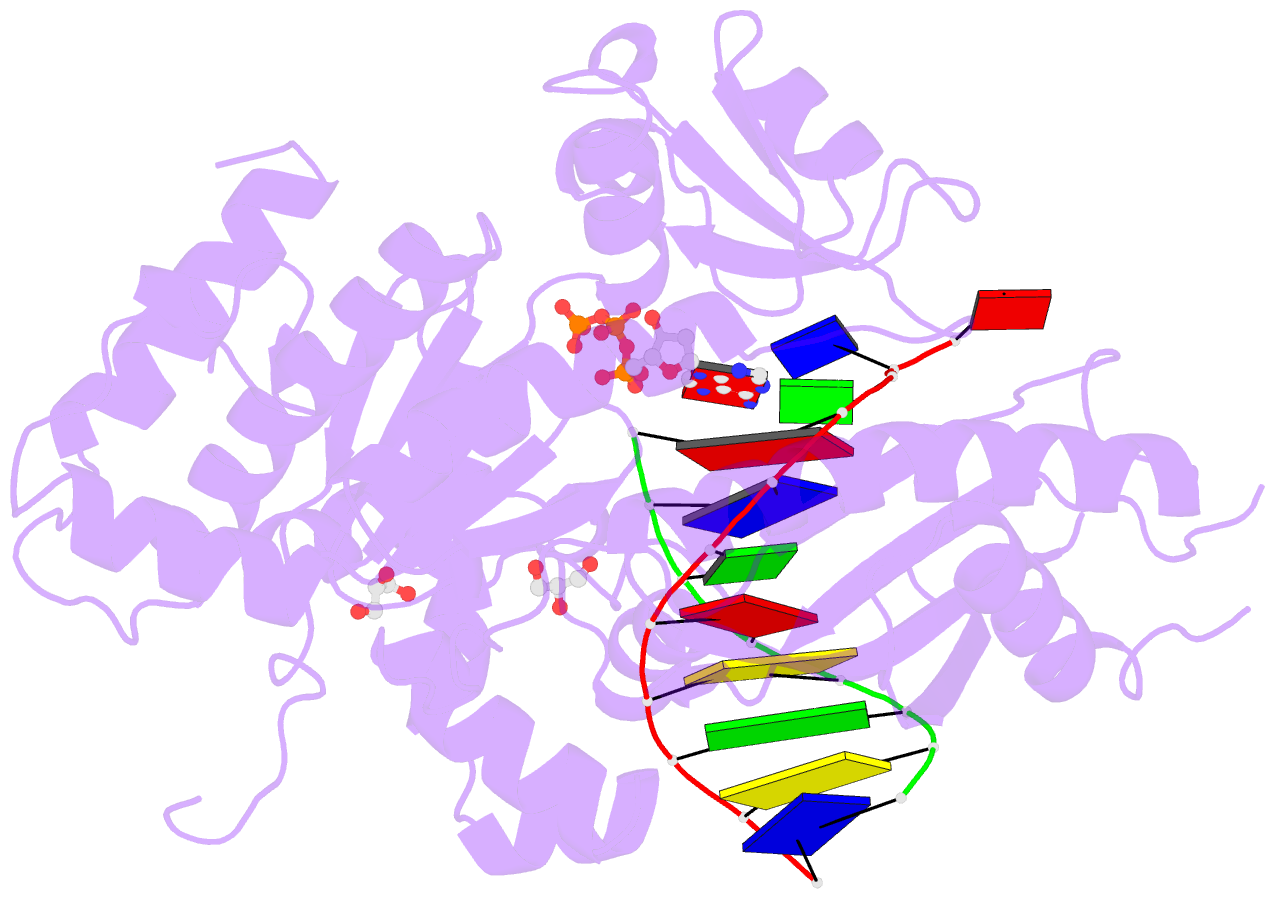
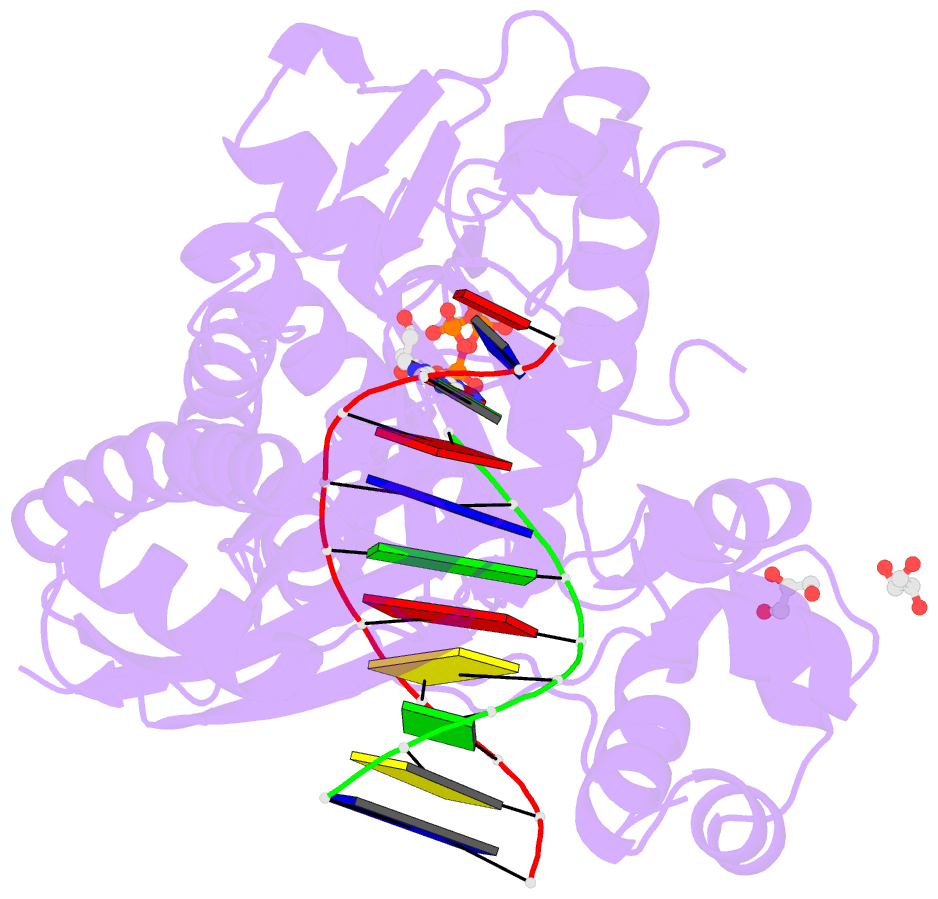
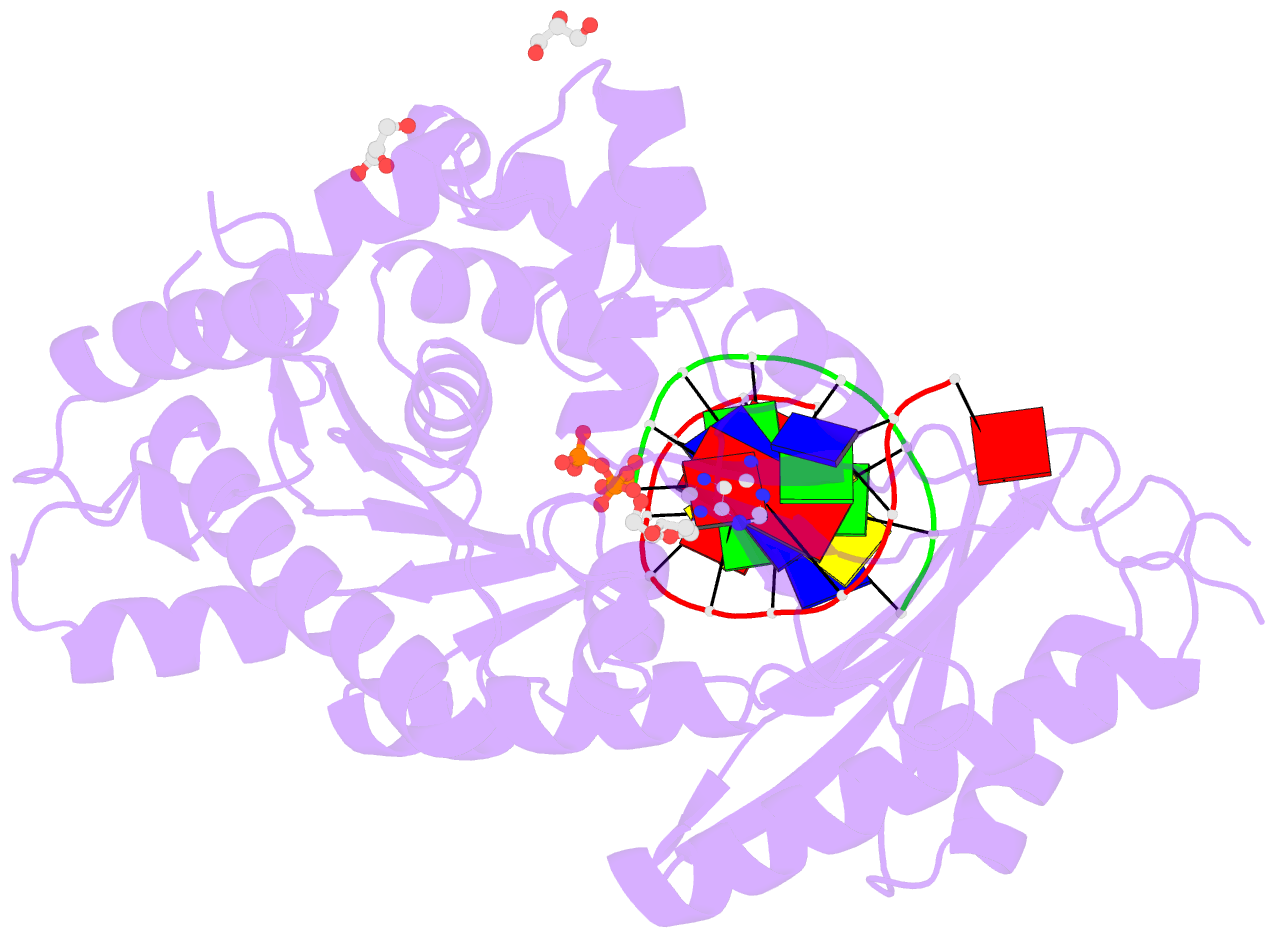
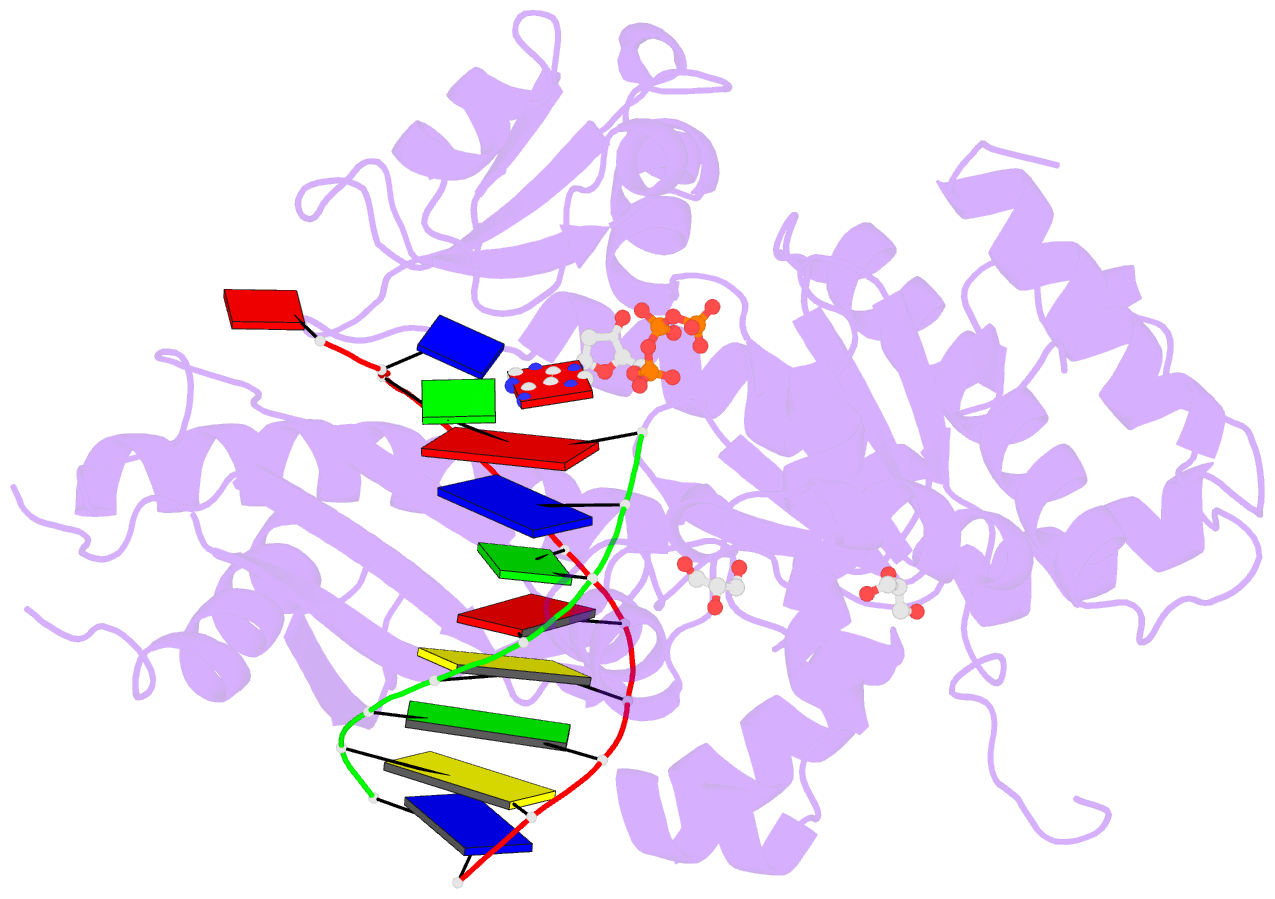
Summary information and primary citation
- PDB-id
- 4yr2; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase-DNA
- Method
- X-ray (1.95 Å)
- Summary
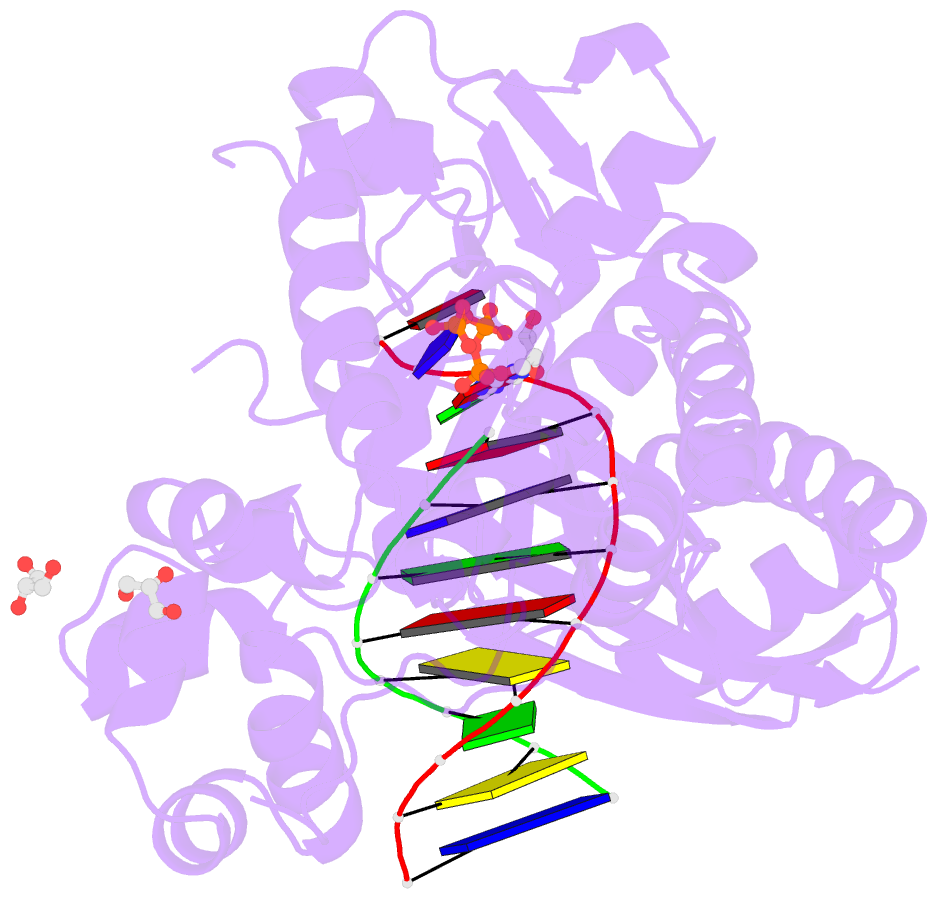
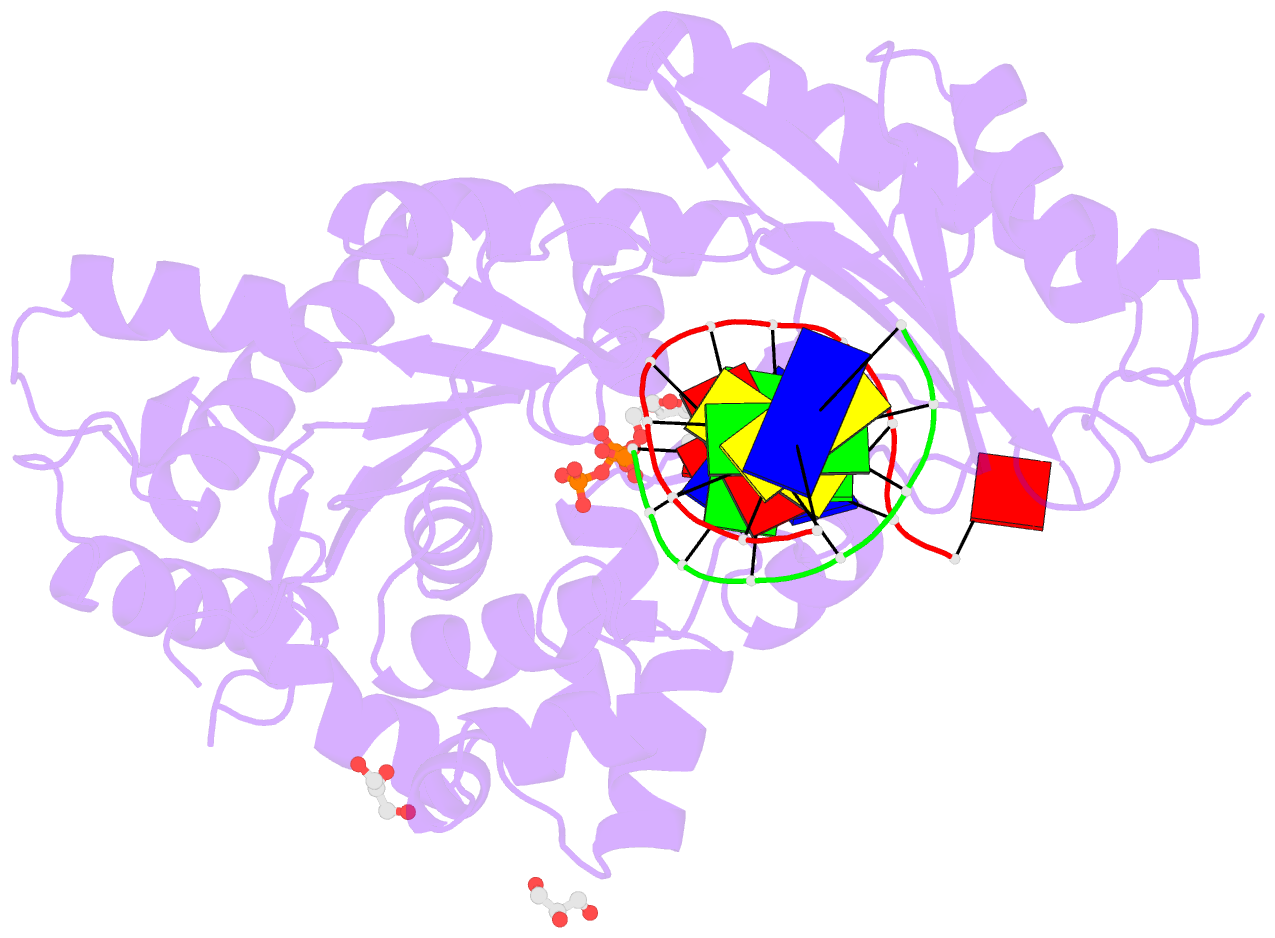
- Mutant human DNA polymerase eta r61m inserting datp opposite an 8-oxoguanine lesion
- Reference
- Su Y, Patra A, Harp JM, Egli M, Guengerich FP (2015): "Roles of Residues Arg-61 and Gln-38 of Human DNA Polymerase eta in Bypass of Deoxyguanosine and 7,8-Dihydro-8-oxo-2'-deoxyguanosine." J.Biol.Chem., 290, 15921-15933. doi: 10.1074/jbc.M115.653691.
- Abstract
- Like the other Y-family DNA polymerases, human DNA polymerase η (hpol η) has relatively low fidelity and is able to tolerate damage during DNA synthesis, including 7,8-dihydro-8-oxo-2'-deoxyguanosine (8-oxoG), one of the most abundant DNA lesions in the genome. Crystal structures show that Arg-61 and Gln-38 are located near the active site and may play important roles in the fidelity and efficiency of hpol η. Site-directed mutagenesis was used to replace these side chains either alone or together, and the wild type or mutant proteins were purified and tested by replicating DNA past deoxyguanosine (G) or 8-oxoG. The catalytic activity of hpol η was dramatically disrupted by the R61M and Q38A/R61A mutations, as opposed to the R61A and Q38A single mutants. Crystal structures of hpol η mutant ternary complexes reveal that polarized water molecules can mimic and partially compensate for the missing side chains of Arg-61 and Gln-38 in the Q38A/R61A mutant. The combined data indicate that the positioning and positive charge of Arg-61 synergistically contribute to the nucleotidyl transfer reaction, with additional influence exerted by Gln-38. In addition, gel filtration chromatography separated multimeric and monomeric forms of wild type and mutant hpol η, indicating the possibility that hpol η forms multimers in vivo.