Summary information and primary citation
- PDB-id
- 4zld; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (1.6 Å)
- Summary
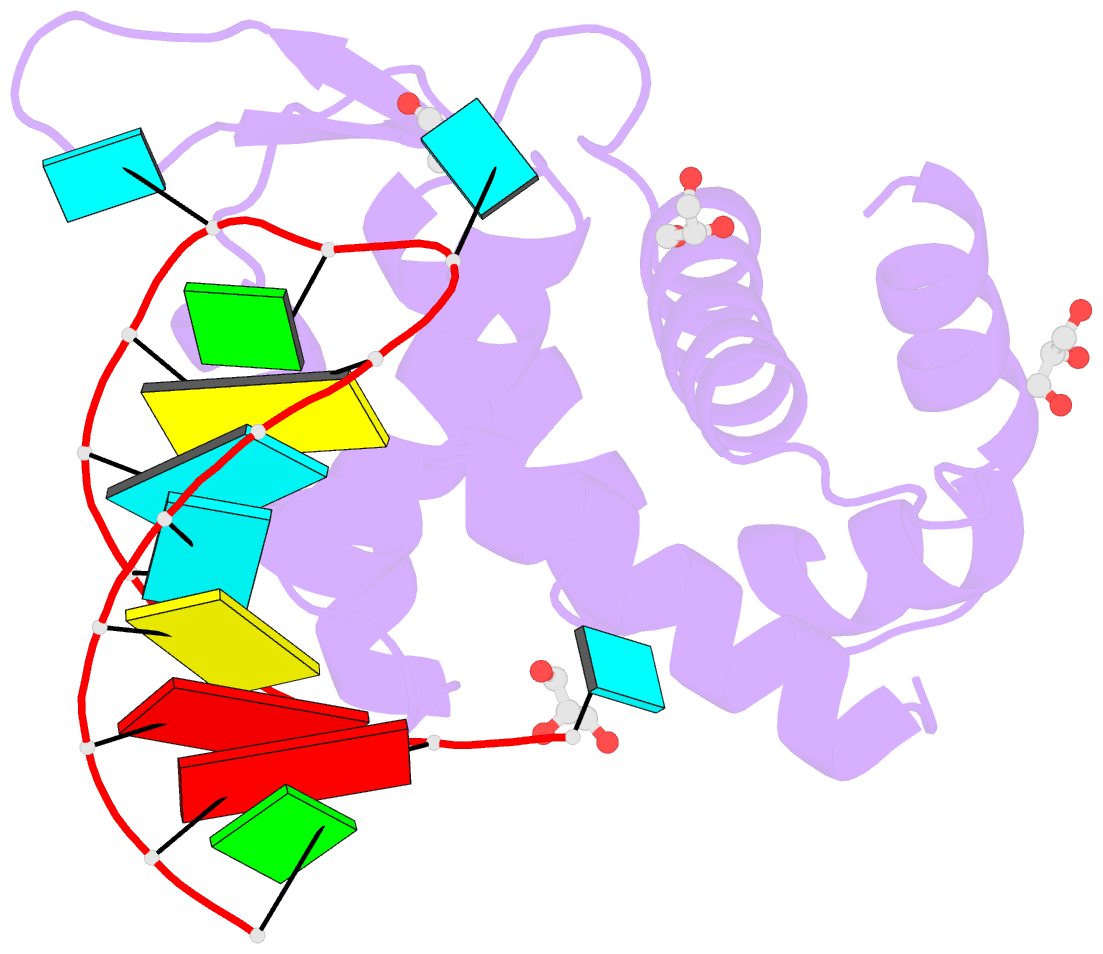
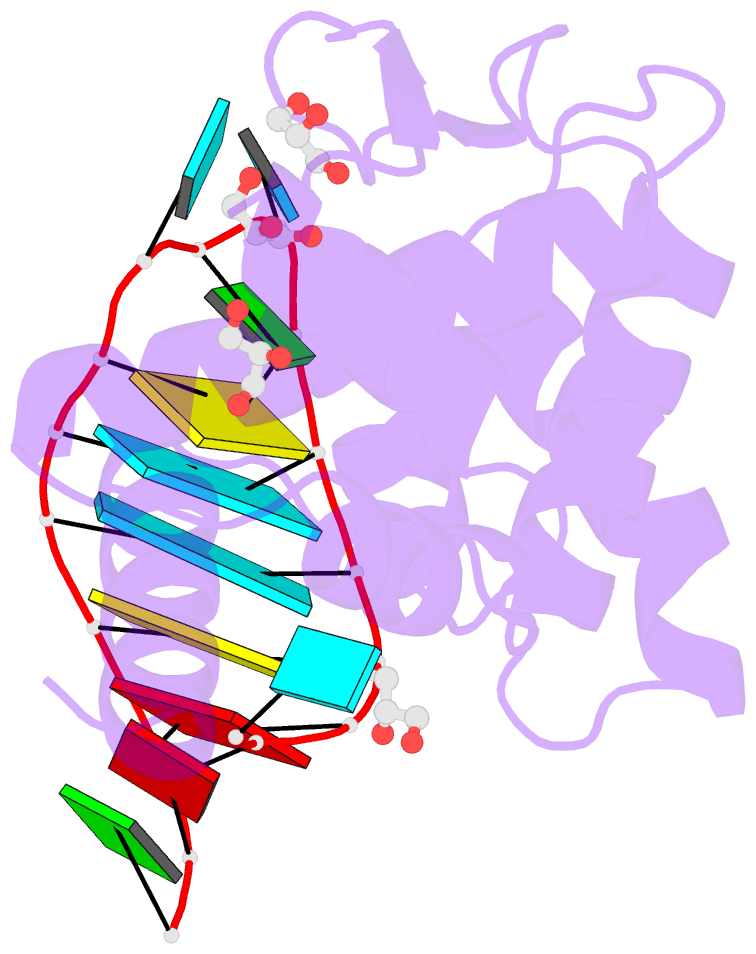
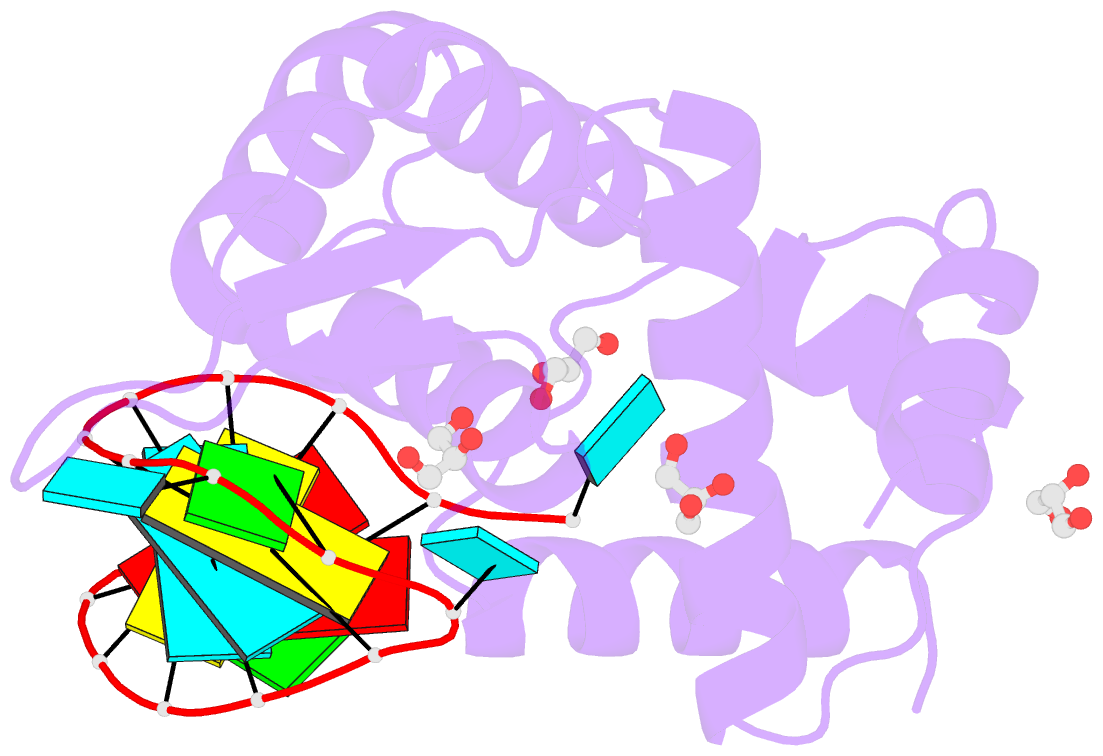
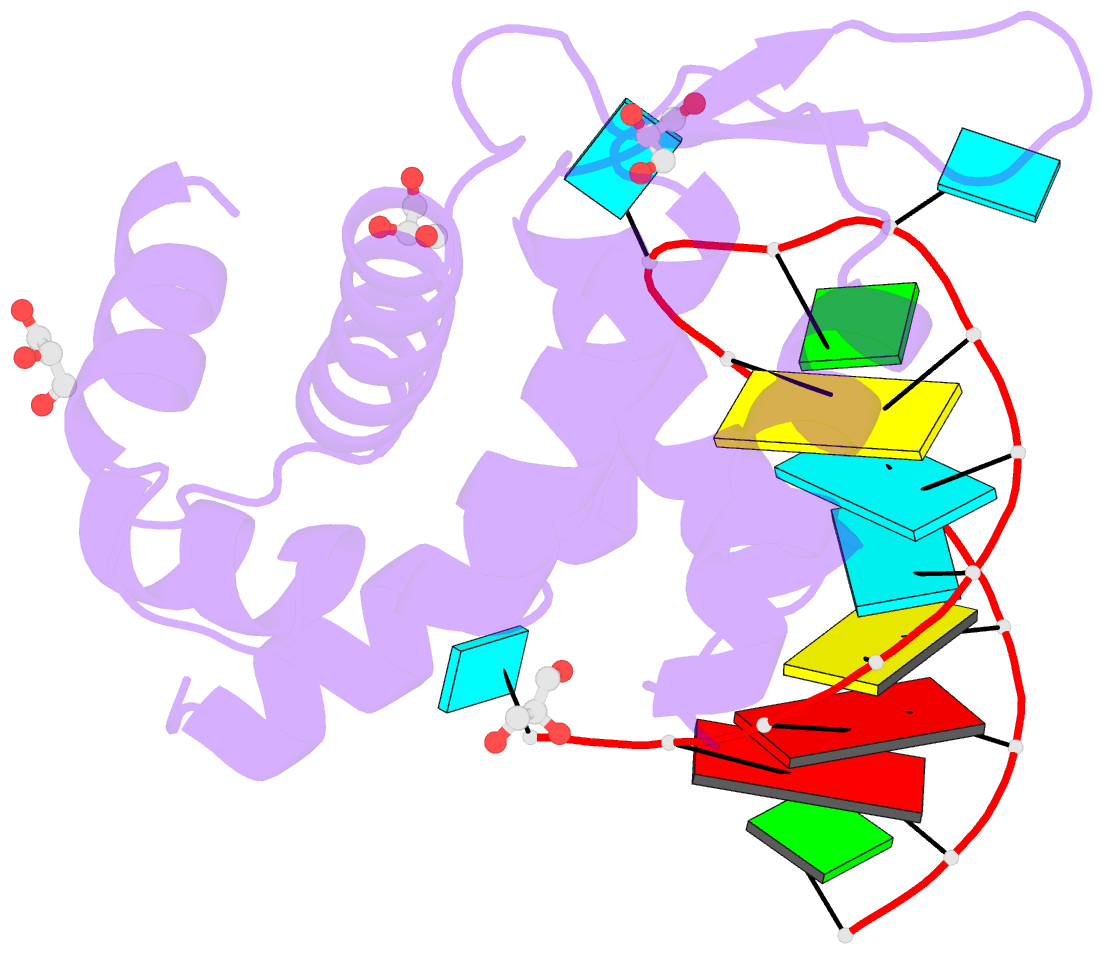
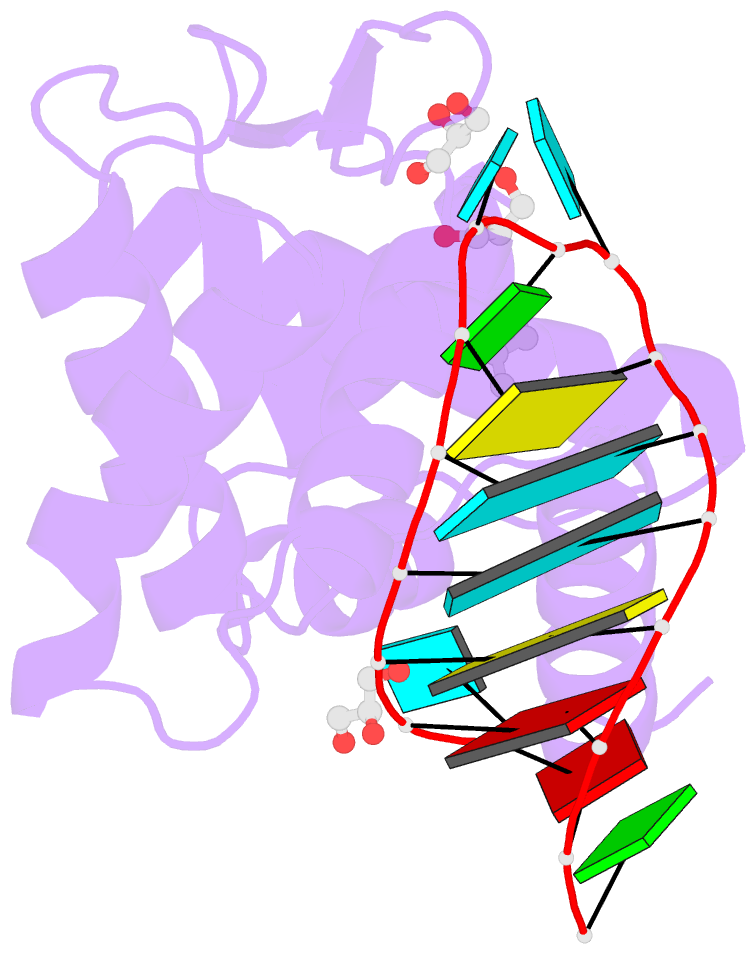
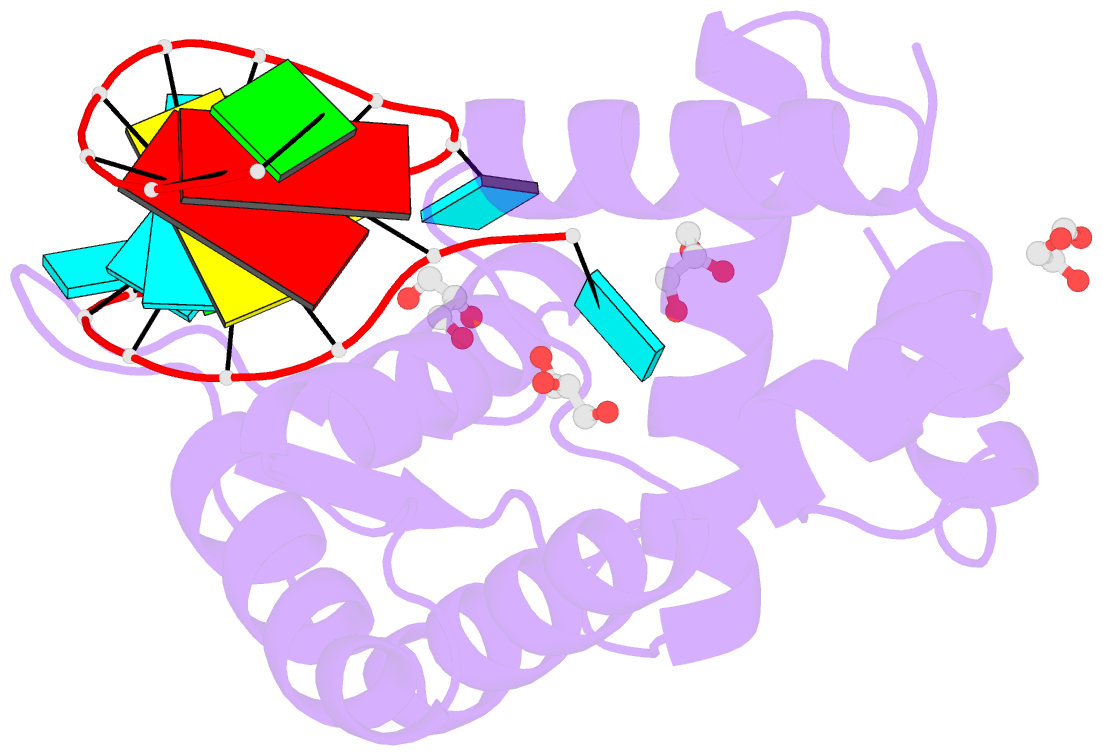
- Crystal structure of human roquin-2 roq domain in complex with roquin cde RNA
- Reference
- Sakurai S, Ohto U, Shimizu T (2015): "Structure of human Roquin-2 and its complex with constitutive-decay element RNA." Acta Crystallogr.,Sect.F, 71, 1048-1054. doi: 10.1107/S2053230X15011887.
- Abstract
- Roquin mediates mRNA degradation by recognizing the constitutive-decay element (CDE) in the 3' untranslated region of the target gene followed by recruitment of the deadenylation machinery. Deficiency or dysfunction of Roquin has been associated with autoimmunity and inflammation. To establish the structural basis for the recognition of CDE RNA by Roquin, the crystal structure of the ROQ domain of human Roquin-2 was determined in ligand-free and CDE-derived RNA-bound forms. The ROQ domain of Roquin-2 folded into a winged-helix structure in which the wing region showed structural flexibility and acted as a lid for RNA binding. The CDE RNA, forming a stem-loop structure, bound to the positively charged surface of the ROQ domain and was mainly recognized via direct interactions with the phosphate backbone in the 5' half of the stem-loop and its triloop and via indirect water-mediated interactions. Structural comparison with Roquin-1 revealed conserved features of the RNA-binding mode. Therefore, it is suggested that the Roquin proteins function redundantly in mRNA degradation.