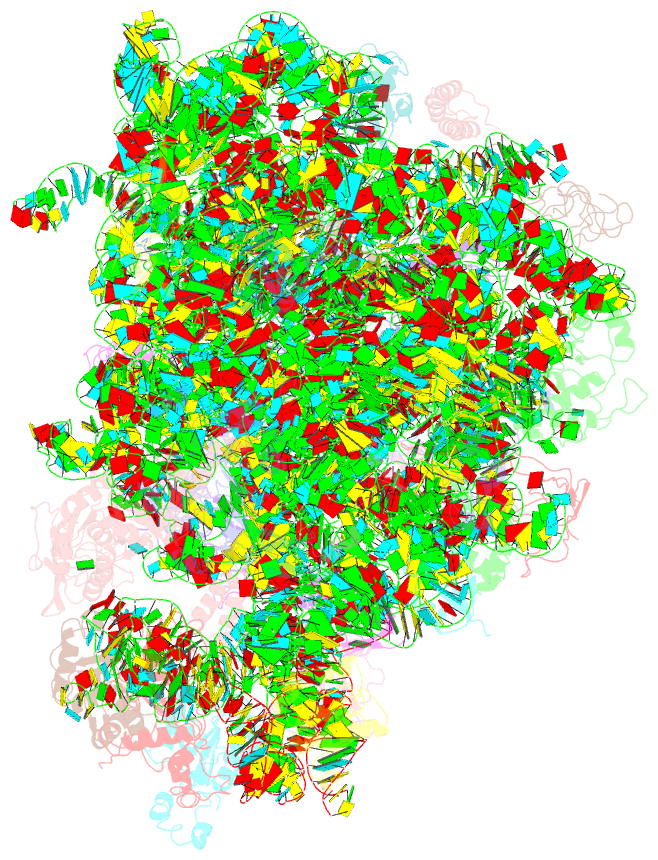
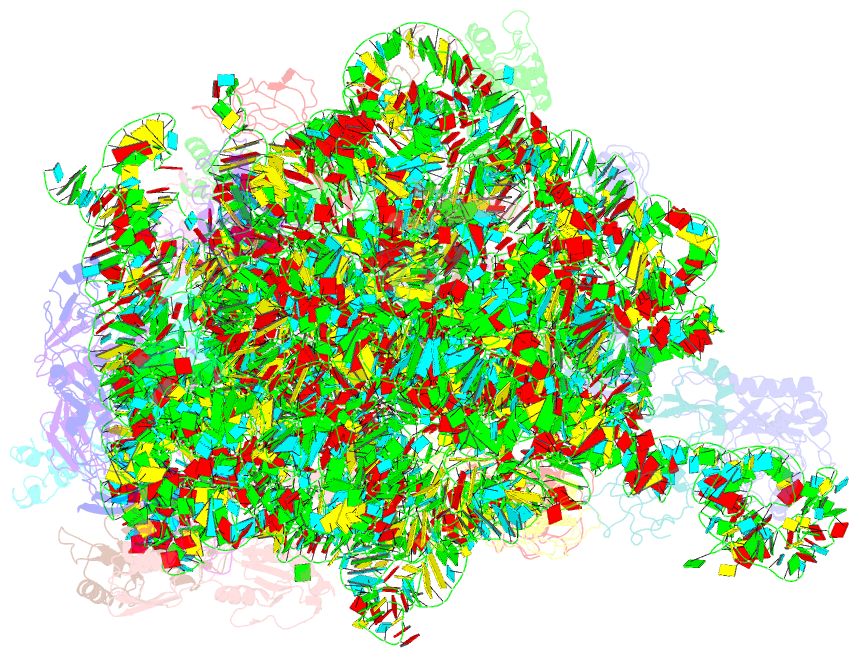
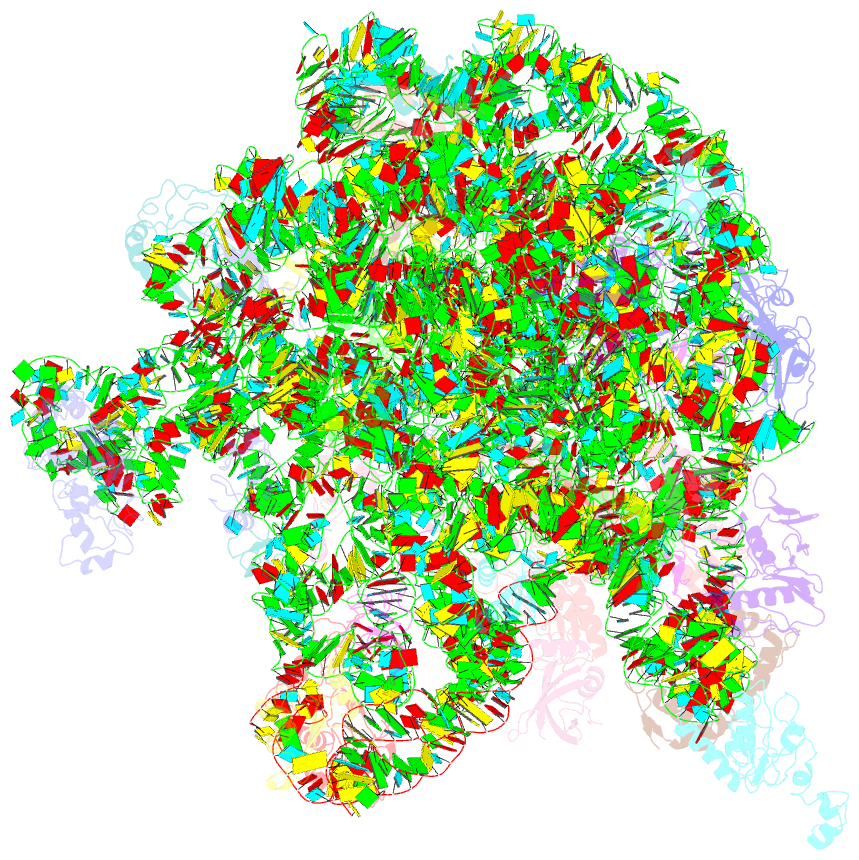
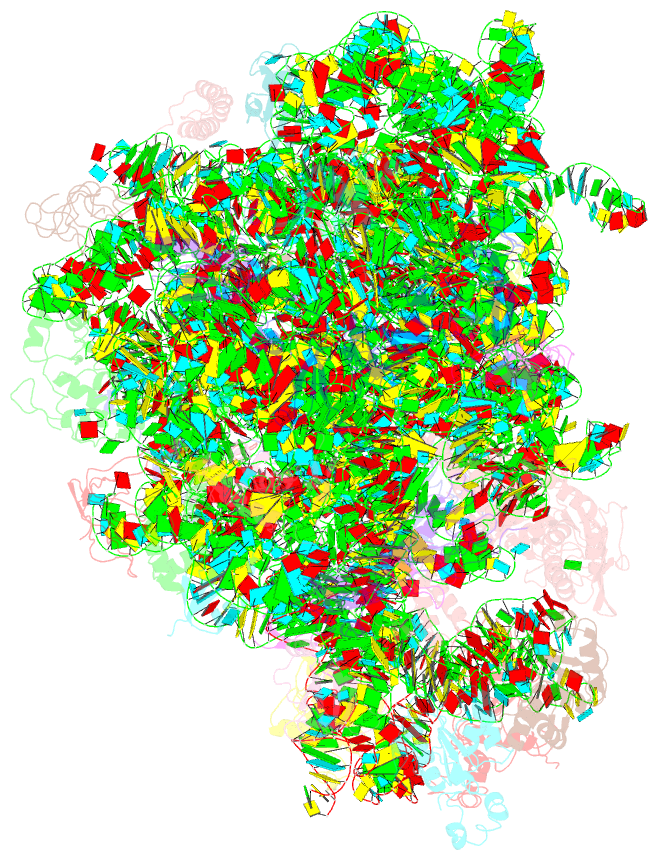
Summary information and primary citation
- PDB-id
- 5ady; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- cryo-EM (4.5 Å)
- Summary
- cryo-EM structures of the 50s ribosome subunit bound with hflx
- Reference
- Zhang Y, Mandava CS, Cao W, Li X, Zhang D, Li N, Zhang Y, Zhang X, Qin Y, Mi K, Lei J, Sanyal S, Gao N (2015): "Hflx is a Ribosome Splitting Factor Rescuing Stalled Ribosomes Under Stress Conditions." Nat.Struct.Mol.Biol., 22, 906. doi: 10.1038/NSMB.3103.
- Abstract
- Adverse cellular conditions often lead to nonproductive translational stalling and arrest of ribosomes on mRNAs. Here, we used fast kinetics and cryo-EM to characterize Escherichia coli HflX, a GTPase with unknown function. Our data reveal that HflX is a heat shock-induced ribosome-splitting factor capable of dissociating vacant as well as mRNA-associated ribosomes with deacylated tRNA in the peptidyl site. Structural data demonstrate that the N-terminal effector domain of HflX binds to the peptidyl transferase center in a strikingly similar manner as that of the class I release factors and induces dramatic conformational changes in central intersubunit bridges, thus promoting subunit dissociation. Accordingly, loss of HflX results in an increase in stalled ribosomes upon heat shock. These results suggest a primary role of HflX in rescuing translationally arrested ribosomes under stress conditions.