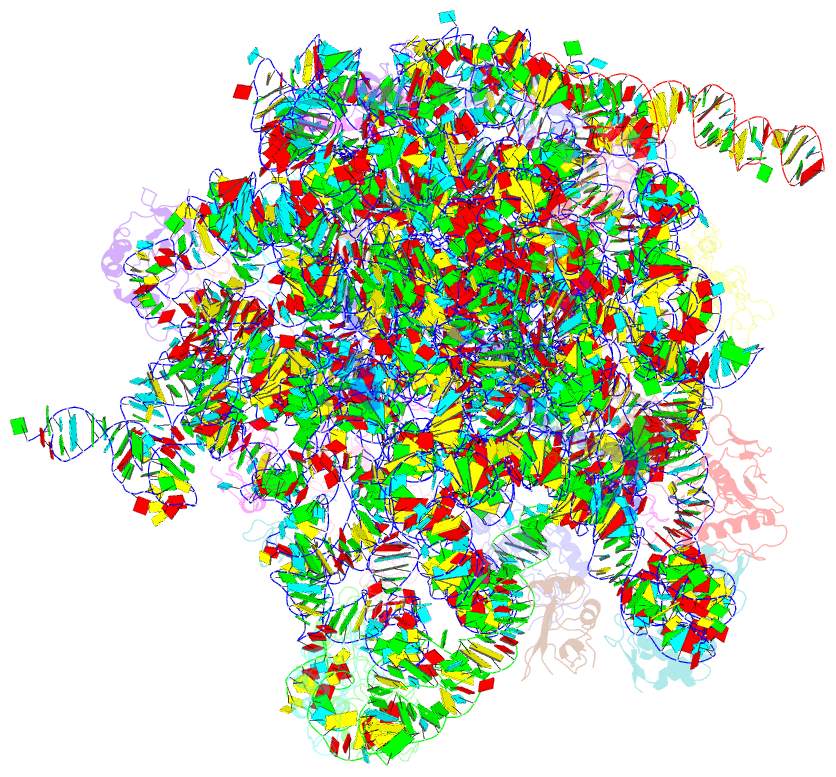
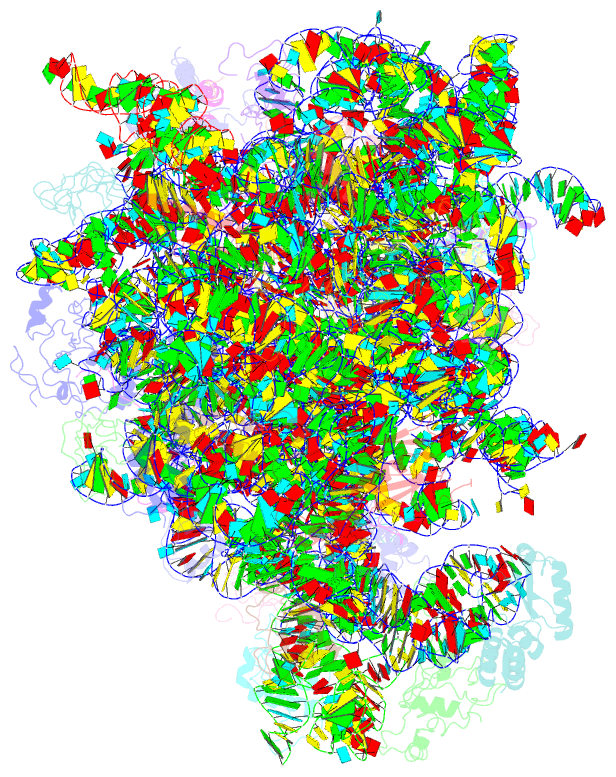
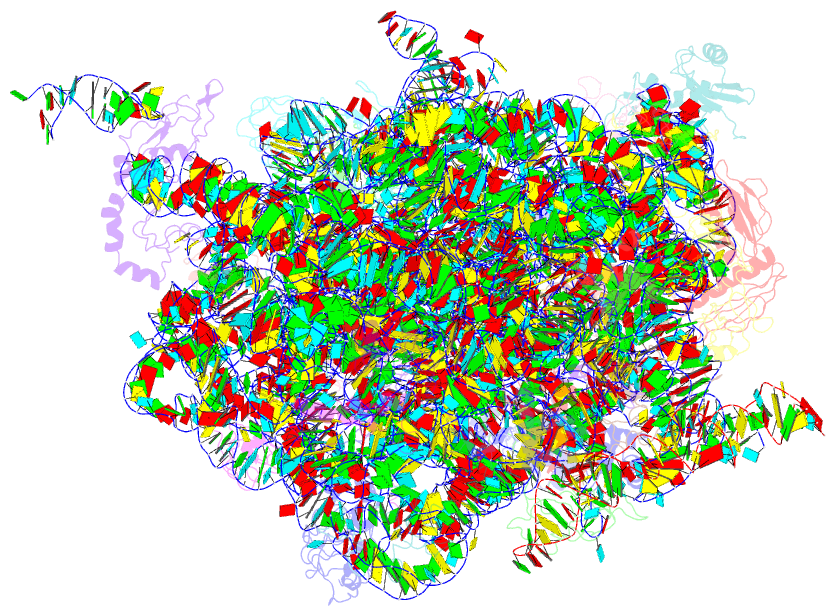
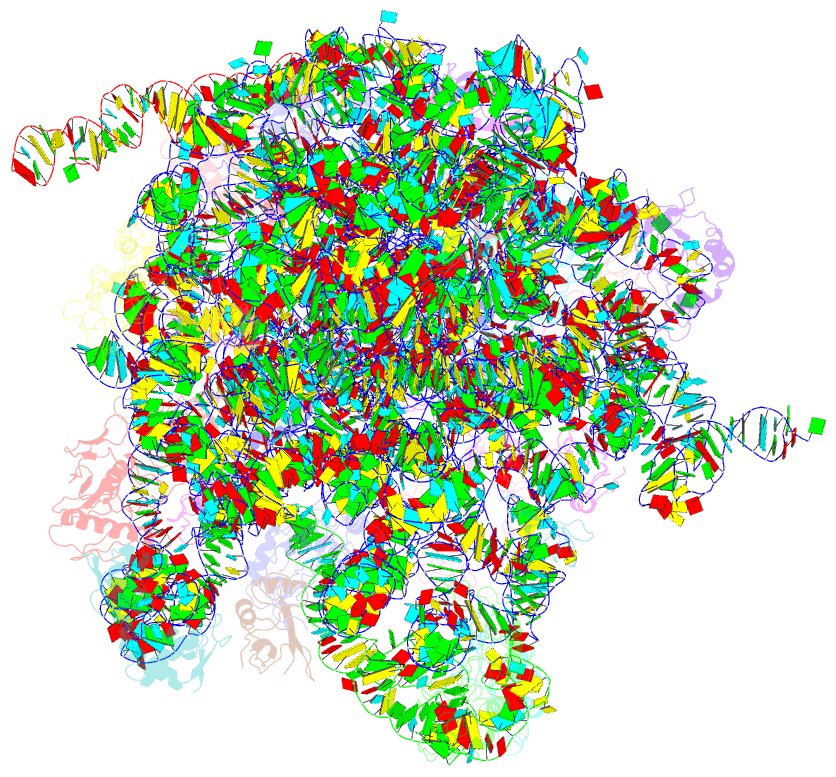
Summary information and primary citation
- PDB-id
- 5aka; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- cryo-EM (5.7 Å)
- Summary
- Em structure of ribosome-srp-ftsy complex in closed state
- Reference
- von Loeffelholz O, Jiang Q, Ariosa A, Karuppasamy M, Huard K, Berger I, Shan S, Schaffitzel C (2015): "Ribosome-Srp-Ftsy Cotranslational Targeting Complex in the Closed State." Proc.Natl.Acad.Sci.USA, 112, 3943-3948. doi: 10.1073/PNAS.1424453112.
- Abstract
- The signal recognition particle (SRP)-dependent pathway is essential for correct targeting of proteins to the membrane and subsequent insertion in the membrane or secretion. In Escherichia coli, the SRP and its receptor FtsY bind to ribosome-nascent chain complexes with signal sequences and undergo a series of distinct conformational changes, which ensures accurate timing and fidelity of protein targeting. Initial recruitment of the SRP receptor FtsY to the SRP-RNC complex results in GTP-independent binding of the SRP-FtsY GTPases at the SRP RNA tetraloop. In the presence of GTP, a closed state is adopted by the SRP-FtsY complex. The cryo-EM structure of the closed state reveals an ordered SRP RNA and SRP M domain with a signal sequence-bound. Van der Waals interactions between the finger loop and ribosomal protein L24 lead to a constricted signal sequence-binding pocket possibly preventing premature release of the signal sequence. Conserved M-domain residues contact ribosomal RNA helices 24 and 59. The SRP-FtsY GTPases are detached from the RNA tetraloop and flexible, thus liberating the ribosomal exit site for binding of the translocation machinery.