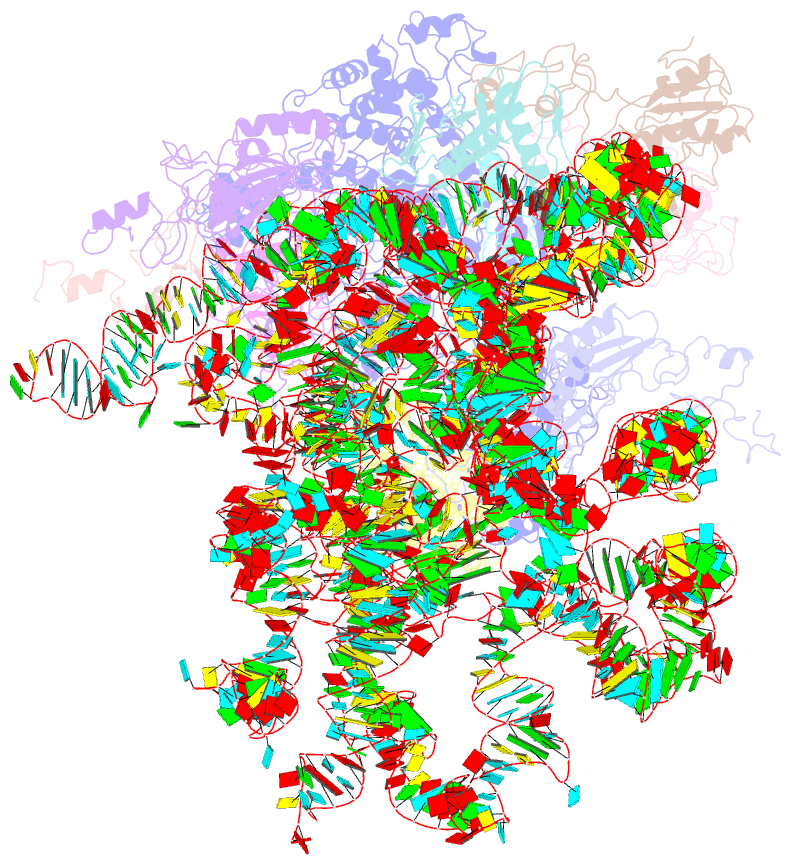
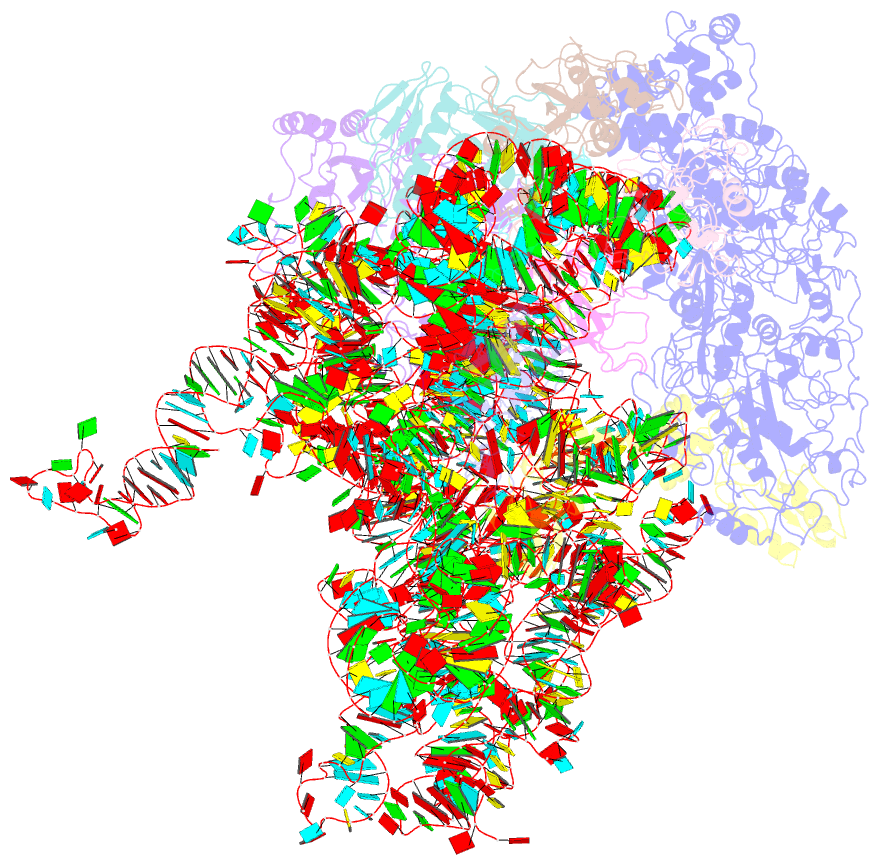
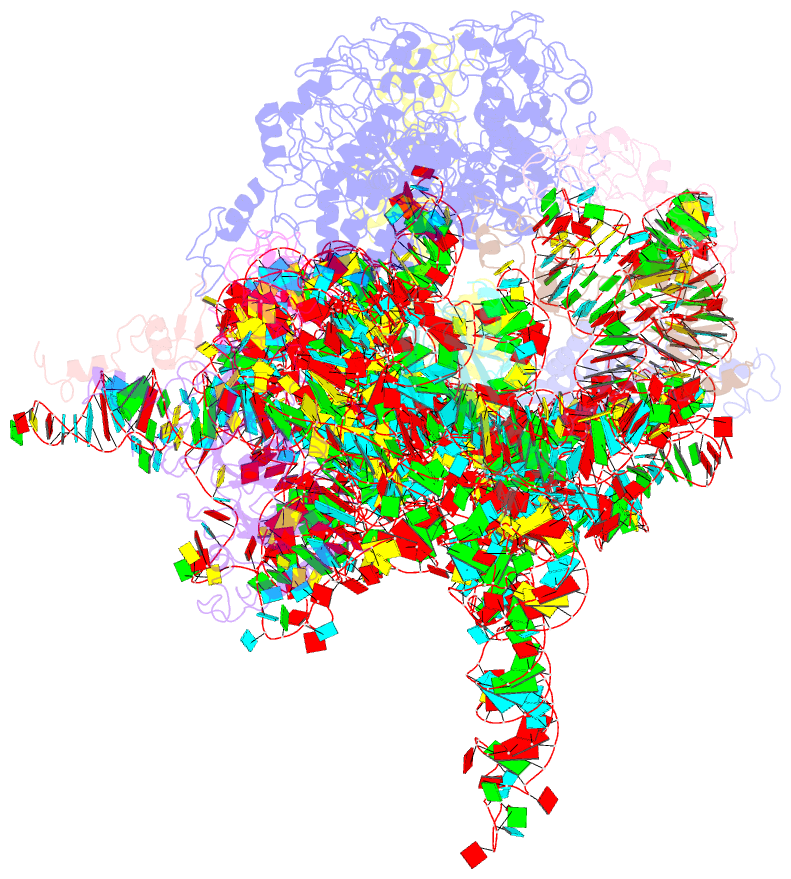
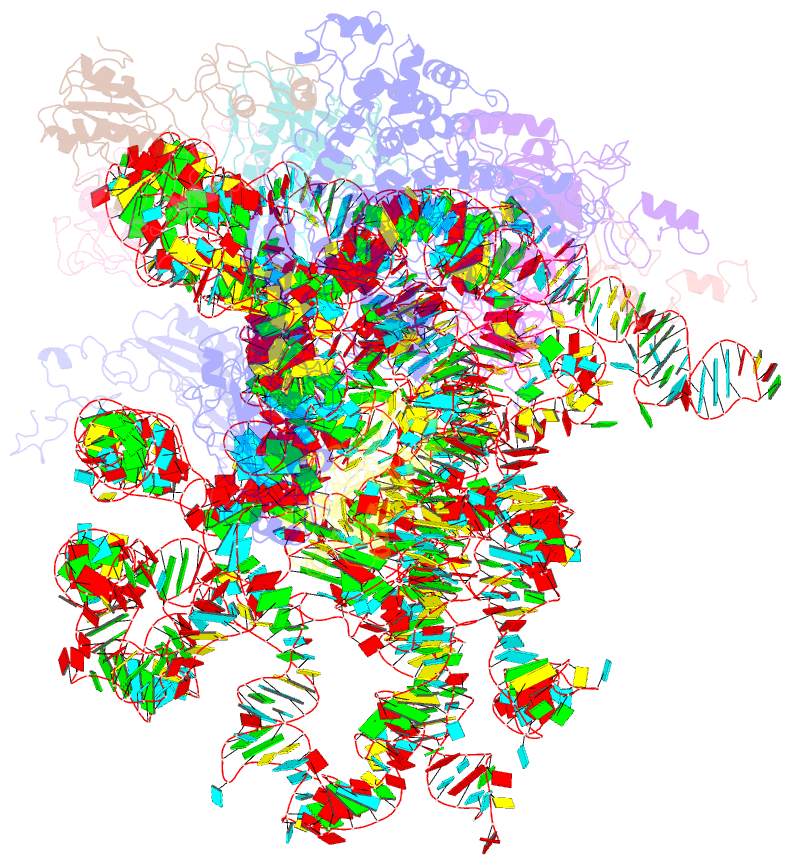
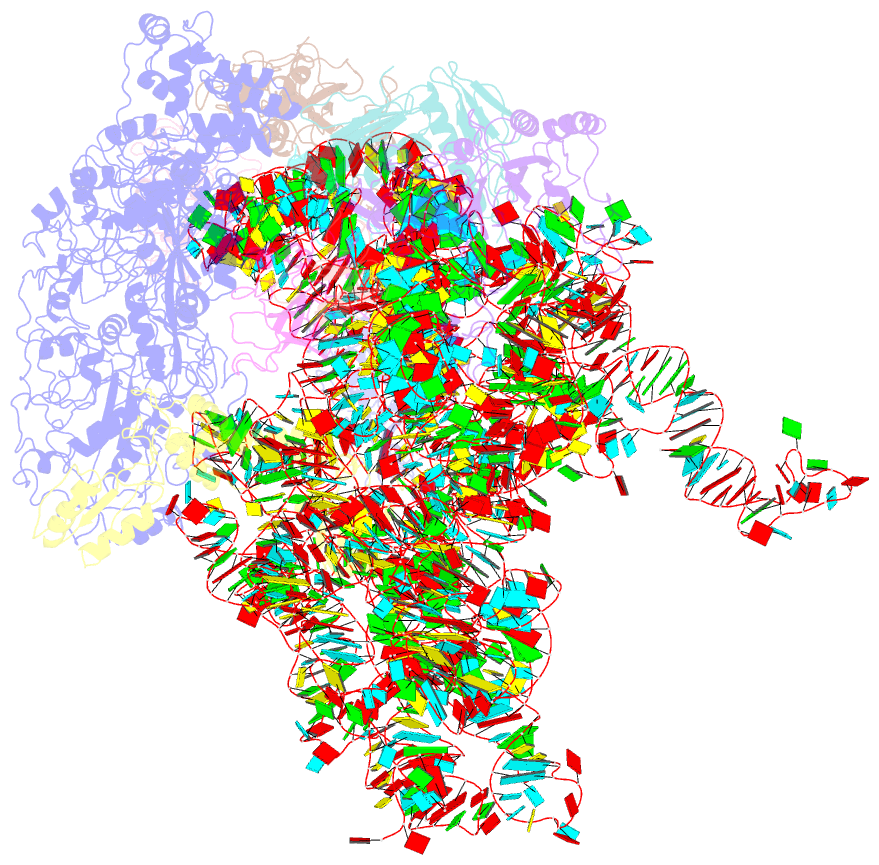
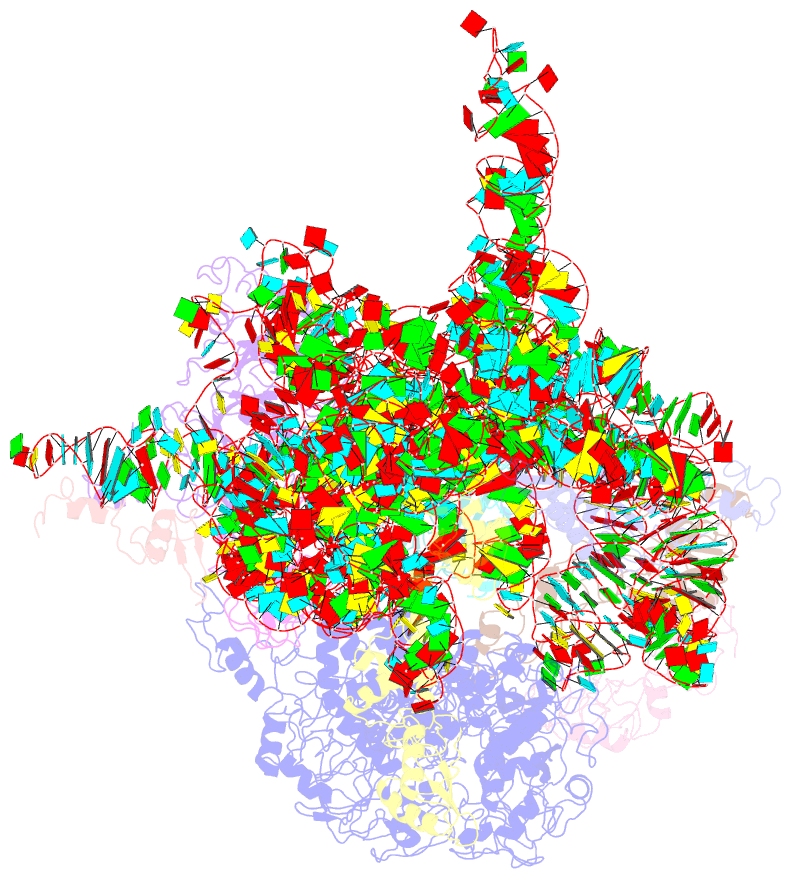
Summary information and primary citation
- PDB-id
- 5anc; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- translation
- Method
- cryo-EM (4.2 Å)
- Summary
- Mechanism of eif6 release from the nascent 60s ribosomal subunit
- Reference
- Weis F, Giudice E, Churcher M, Jin L, Hilcenko C, Wong CC, Traynor D, Kay RR, Warren AJ (2015): "Mechanism of Eif6 Release from the Nascent 60S Ribosomal Subunit." Nat.Struct.Mol.Biol., 22, 914. doi: 10.1038/NSMB.3112.
- Abstract
- SBDS protein (deficient in the inherited leukemia-predisposition disorder Shwachman-Diamond syndrome) and the GTPase EFL1 (an EF-G homolog) activate nascent 60S ribosomal subunits for translation by catalyzing eviction of the antiassociation factor eIF6 from nascent 60S ribosomal subunits. However, the mechanism is completely unknown. Here, we present cryo-EM structures of human SBDS and SBDS-EFL1 bound to Dictyostelium discoideum 60S ribosomal subunits with and without endogenous eIF6. SBDS assesses the integrity of the peptidyl (P) site, bridging uL16 (mutated in T-cell acute lymphoblastic leukemia) with uL11 at the P-stalk base and the sarcin-ricin loop. Upon EFL1 binding, SBDS is repositioned around helix 69, thus facilitating a conformational switch in EFL1 that displaces eIF6 by competing for an overlapping binding site on the 60S ribosomal subunit. Our data reveal the conserved mechanism of eIF6 release, which is corrupted in both inherited and sporadic leukemias.