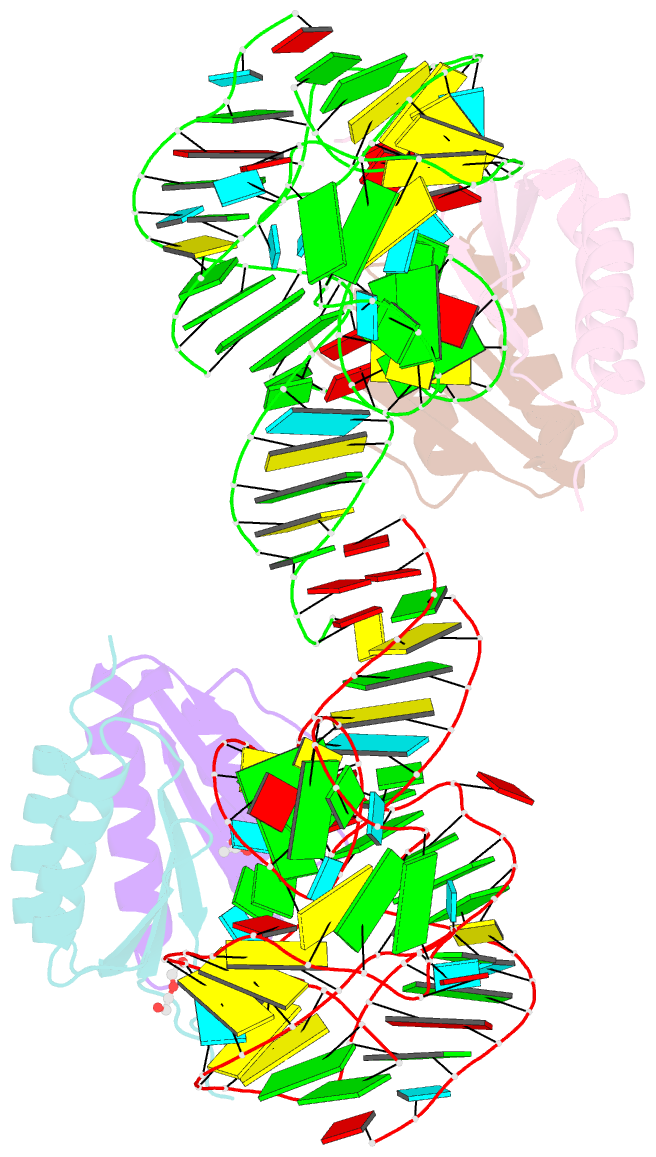
Summary information and primary citation
- PDB-id
- 5aox; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- translation
- Method
- X-ray (2.04 Å)
- Summary
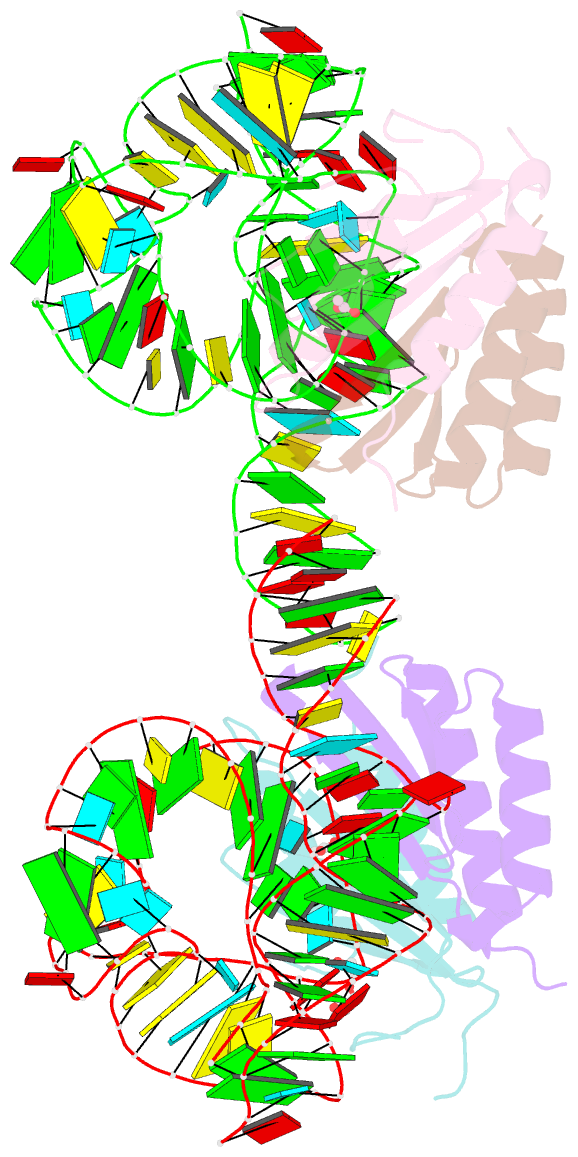
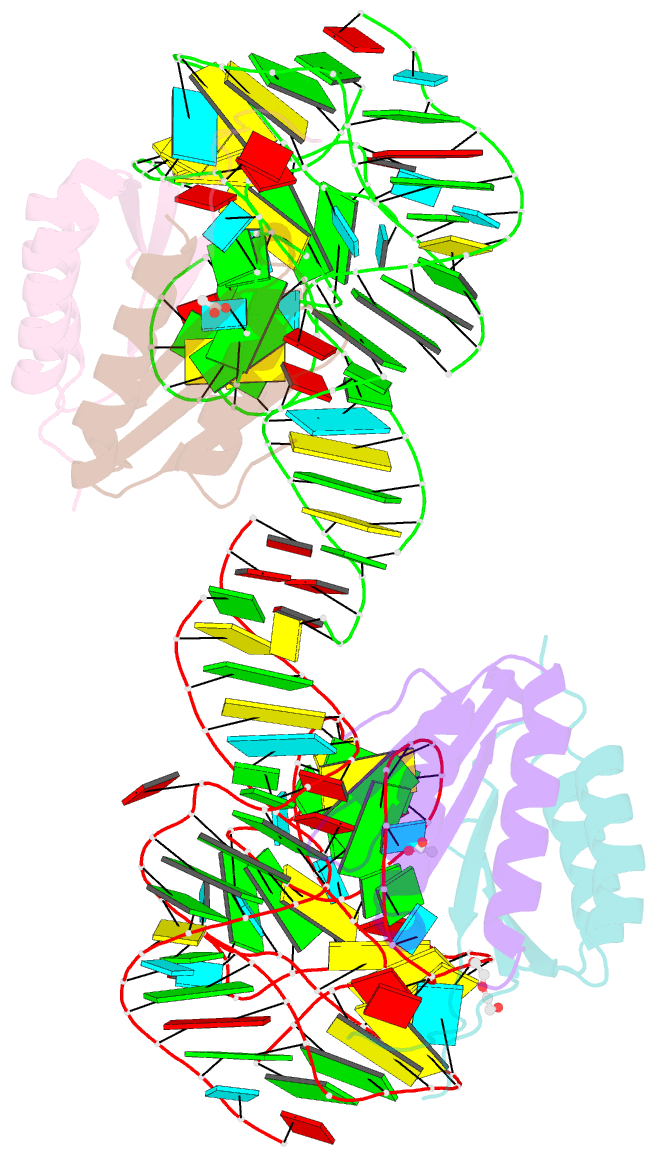
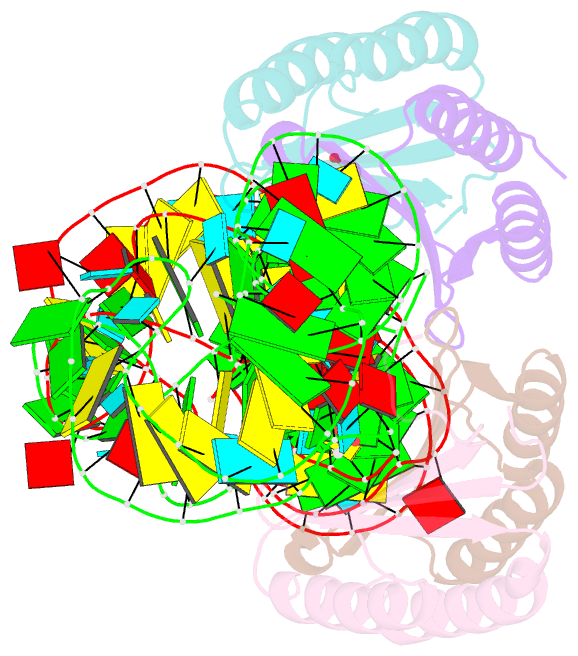
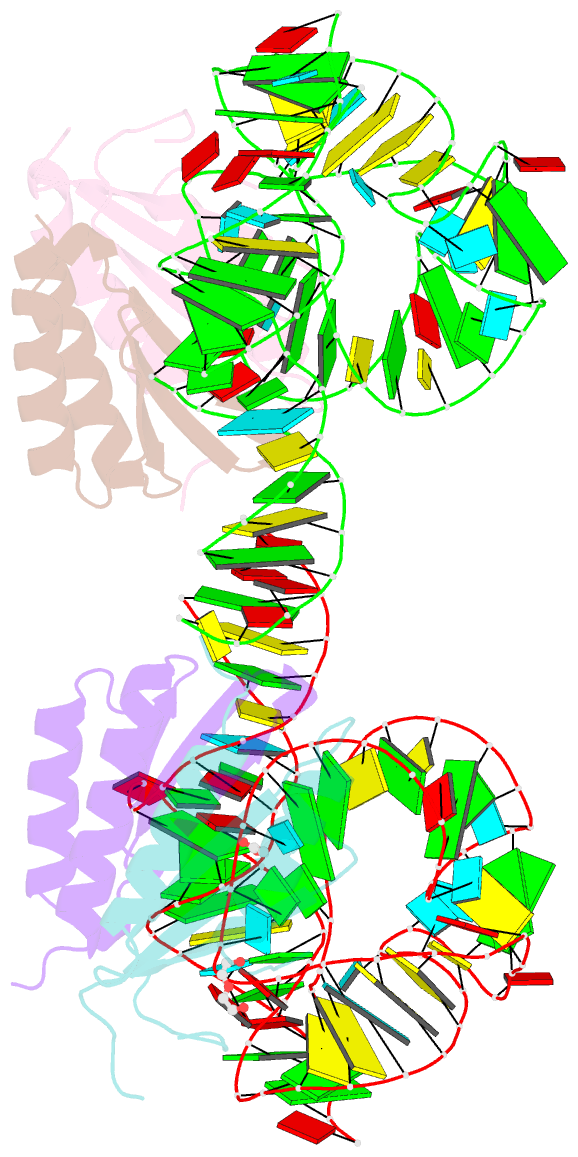
- Human alu RNA retrotransposition complex in the ribosome-stalling conformation
- Reference
- Ahl V, Keller H, Schmidt S, Weichenrieder O (2015): "Retrotransposition and Crystal Structure of an Alu Rnp in the Ribosome-Stalling Conformation." Mol.Cell, 60, 715. doi: 10.1016/J.MOLCEL.2015.10.003.
- Abstract
- The Alu element is the most successful human genomic parasite affecting development and causing disease. It originated as a retrotransposon during early primate evolution of the gene encoding the signal recognition particle (SRP) RNA. We defined a minimal Alu RNA sufficient for effective retrotransposition and determined a high-resolution structure of its complex with the SRP9/14 proteins. The RNA adopts a compact, closed conformation that matches the envelope of the SRP Alu domain in the ribosomal translation elongation factor-binding site. Conserved structural elements in SRP RNAs support an ancient function of the closed conformation that predates SRP9/14. Structure-based mutagenesis shows that retrotransposition requires the closed conformation of the Alu ribonucleoprotein particle and is consistent with the recognition of stalled ribosomes. We propose that ribosome stalling is a common cause for the cis-preference of the mammalian L1 retrotransposon and for the efficiency of the Alu RNA in hijacking nascent L1 reverse transcriptase.