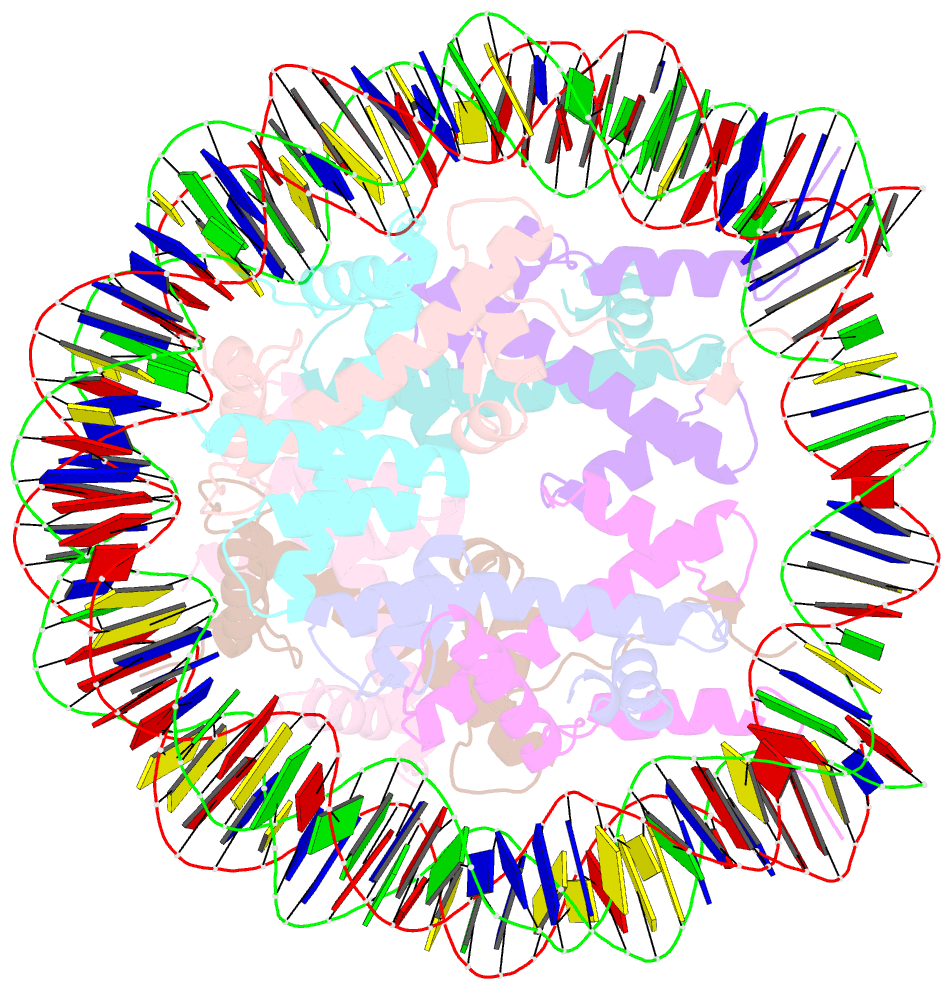
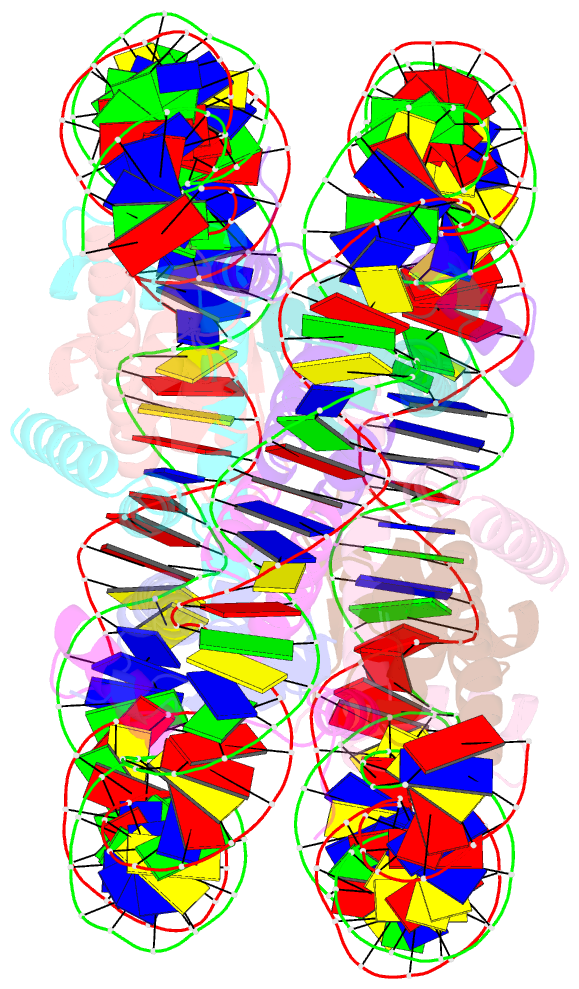
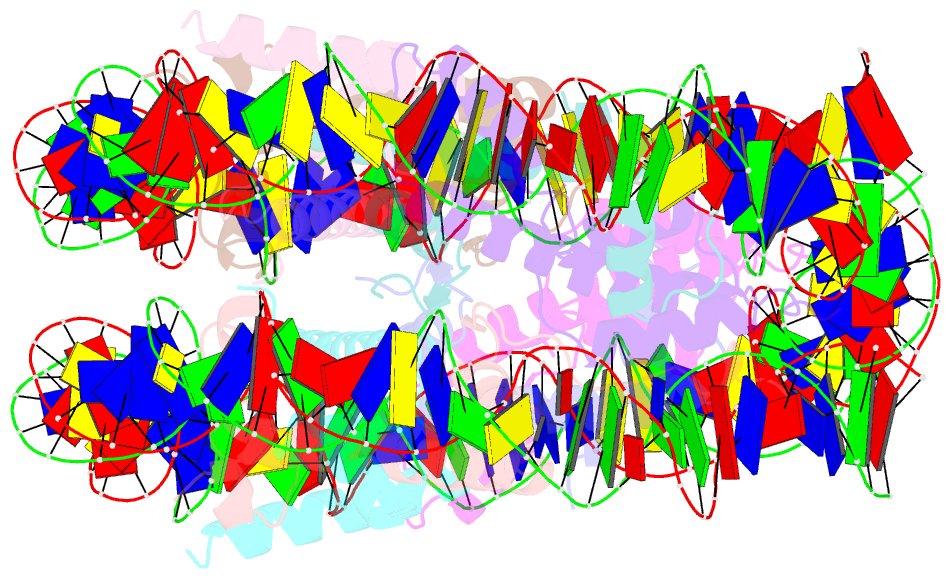
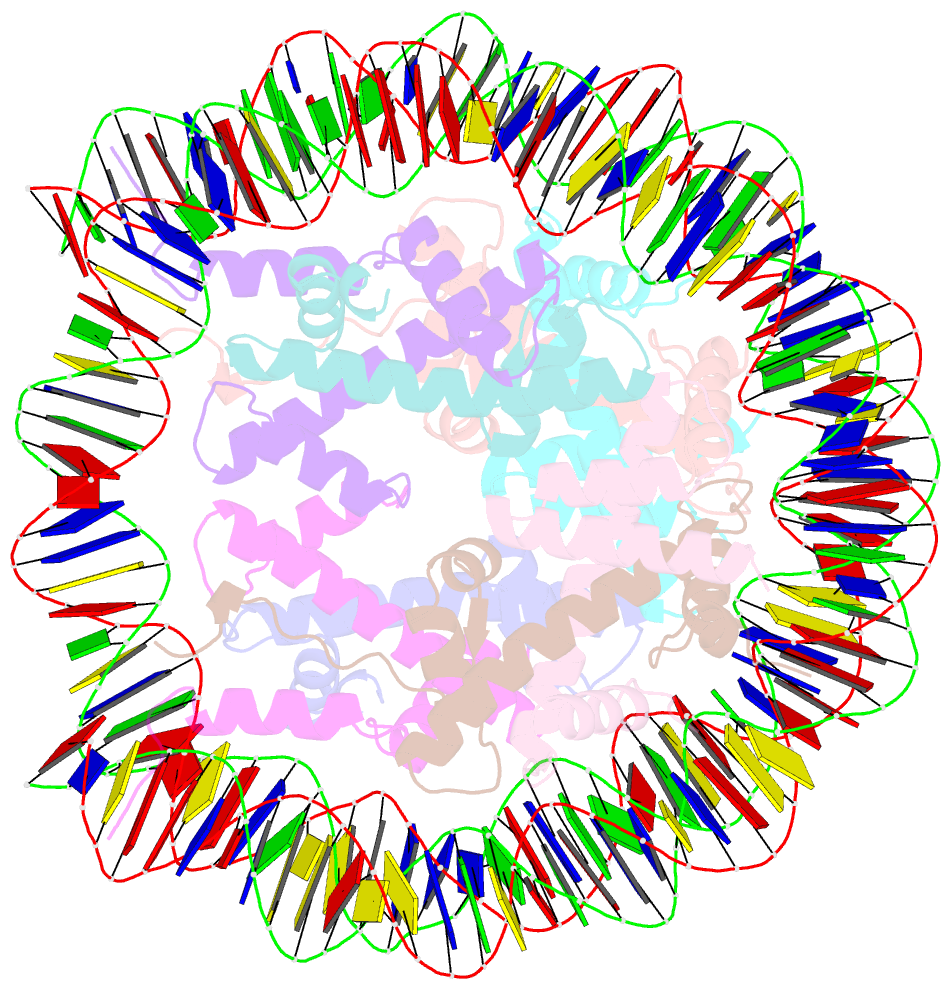
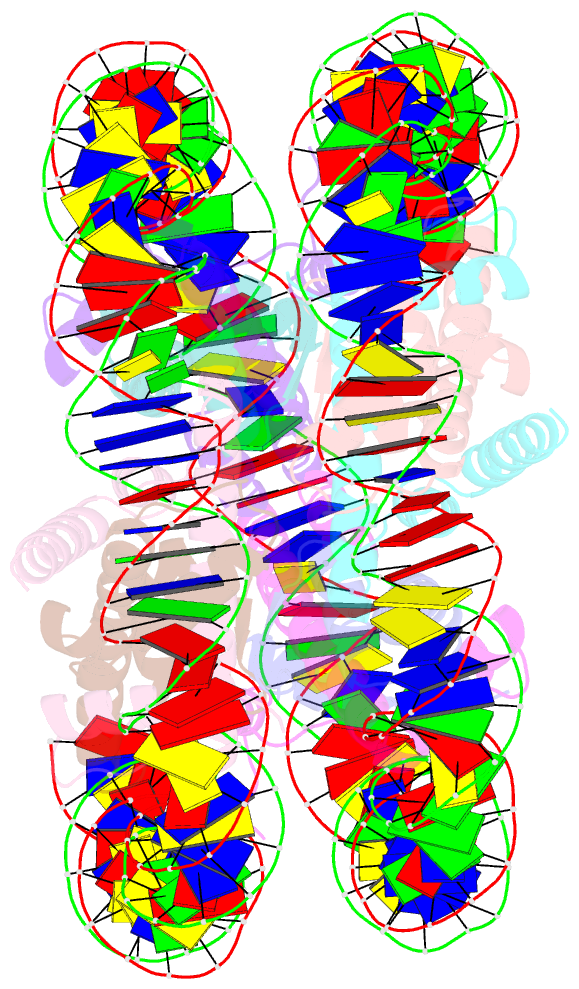
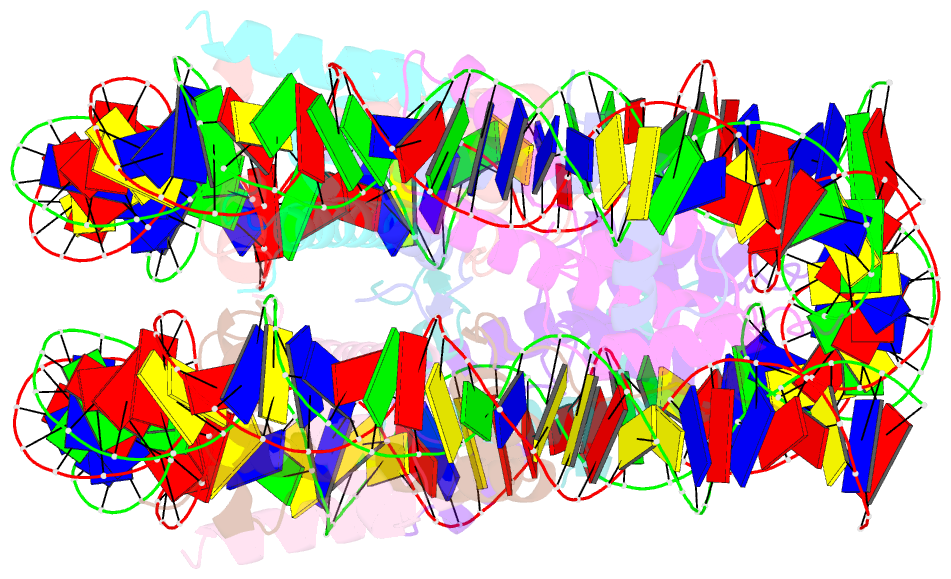
Summary information and primary citation
- PDB-id
- 5ay8; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.8 Å)
- Summary
- Crystal structure of human nucleosome containing h3.y
- Reference
- Kujirai T, Horikoshi N, Sato K, Maehara K, Machida S, Osakabe A, Kimura H, Ohkawa Y, Kurumizaka H (2016): "Structure and function of human histone H3.Y nucleosome." Nucleic Acids Res., 44, 6127-6141. doi: 10.1093/nar/gkw202.
- Abstract
- Histone H3.Y is a primate-specific, distant H3 variant. It is evolutionarily derived from H3.3, and may function in transcription regulation. However, the mechanism by which H3.Y regulates transcription has not been elucidated. In the present study, we determined the crystal structure of the H3.Y nucleosome, and found that many H3.Y-specific residues are located on the entry/exit sites of the nucleosome. Biochemical analyses revealed that the DNA ends of the H3.Y nucleosome were more flexible than those of the H3.3 nucleosome, although the H3.Y nucleosome was stable in vitro and in vivo Interestingly, the linker histone H1, which compacts nucleosomal DNA, appears to bind to the H3.Y nucleosome less efficiently, as compared to the H3.3 nucleosome. These characteristics of the H3.Y nucleosome are also conserved in the H3.Y/H3.3 heterotypic nucleosome, which may be the predominant form in cells. In human cells, H3.Y preferentially accumulated around transcription start sites (TSSs). Taken together, H3.Y-containing nucleosomes around transcription start sites may form relaxed chromatin that allows transcription factor access, to regulate the transcription status of specific genes.