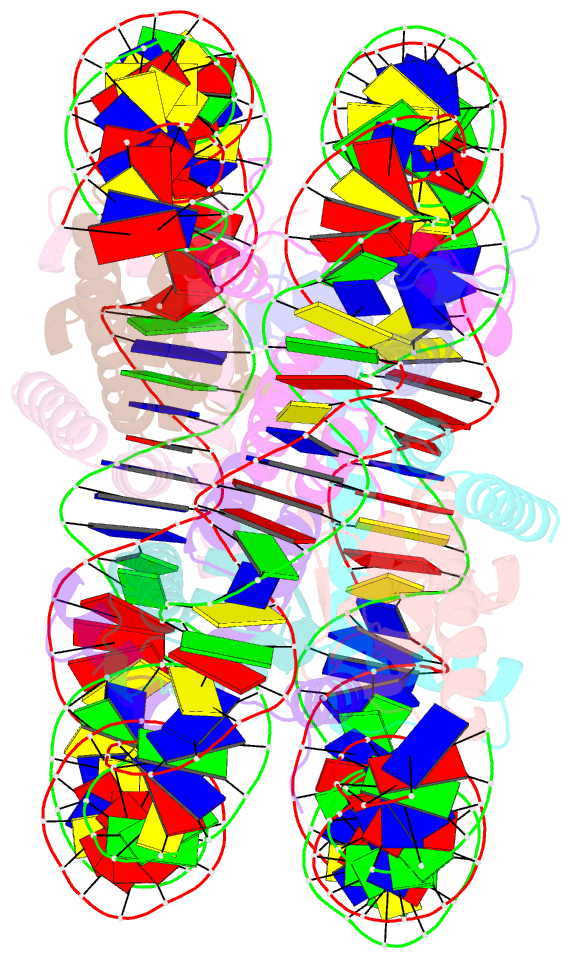
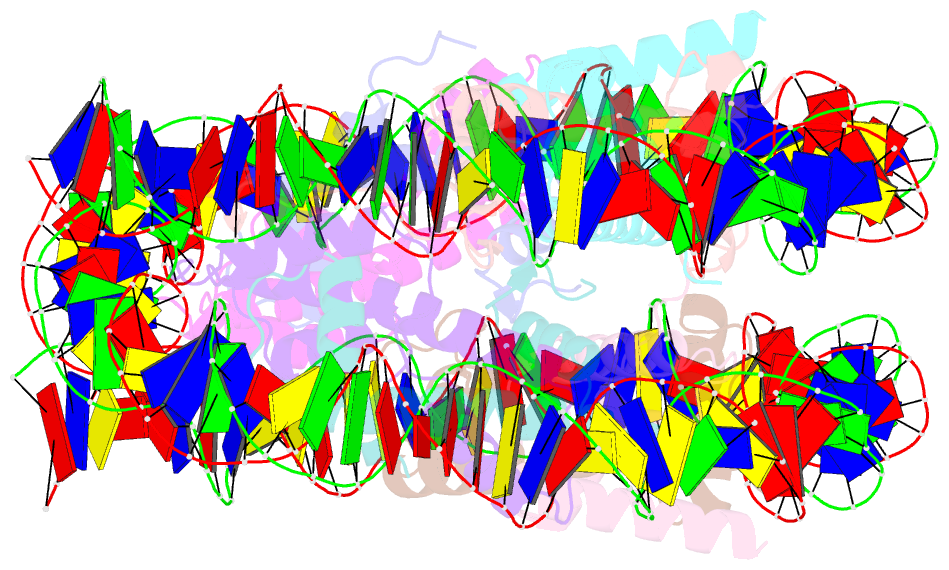
Summary information and primary citation
- PDB-id
- 5b0y; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein
- Method
- X-ray (2.557 Å)
- Summary
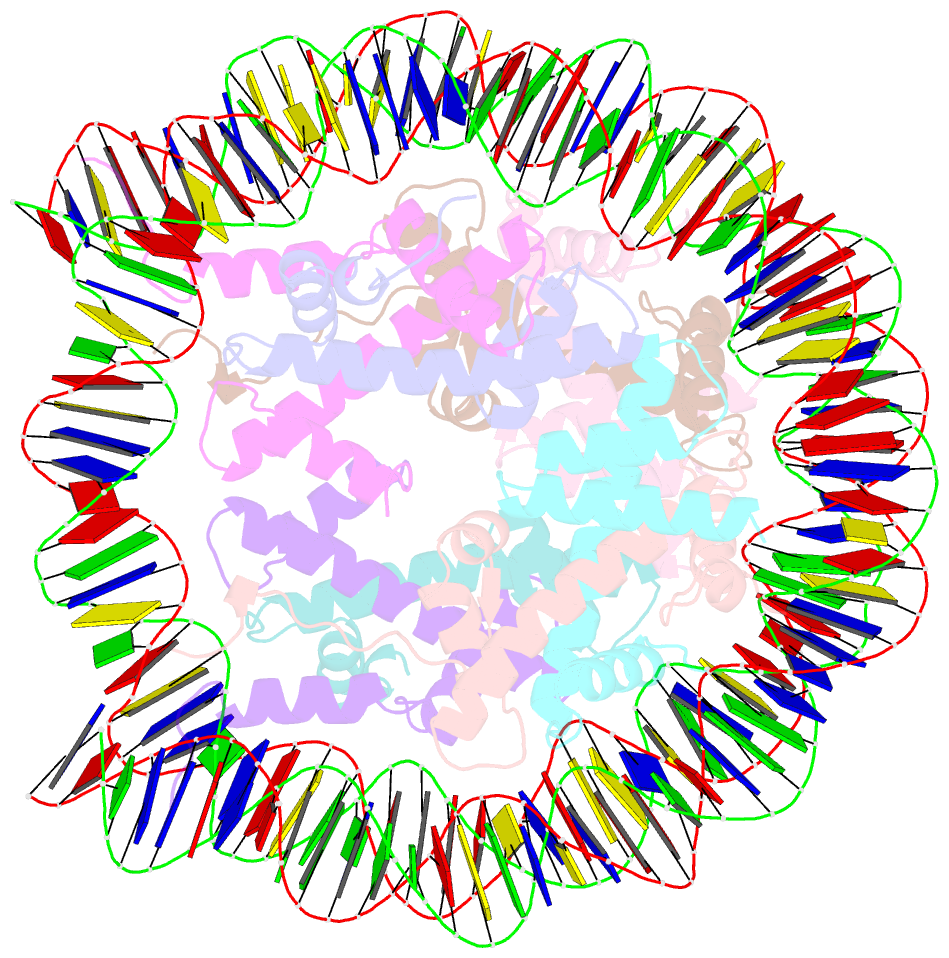
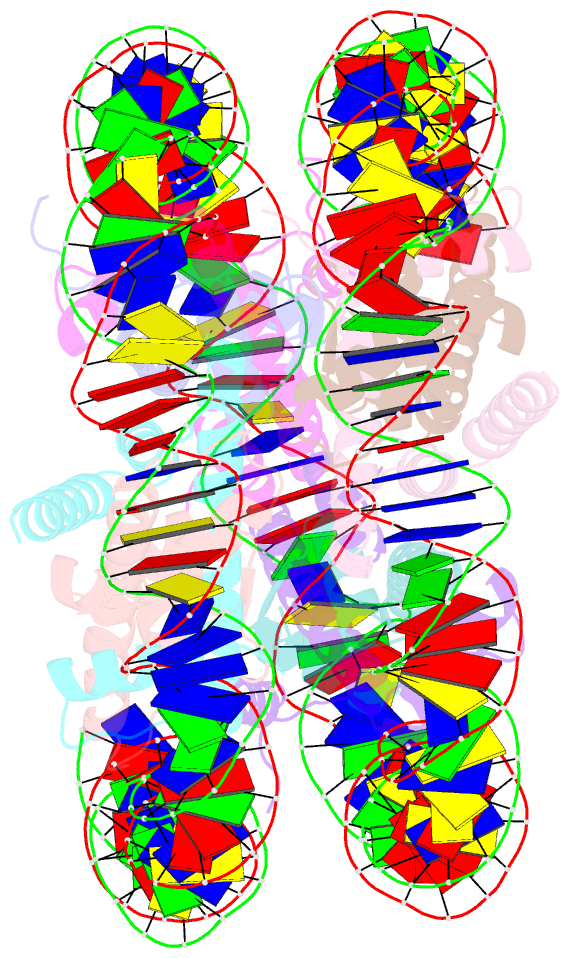
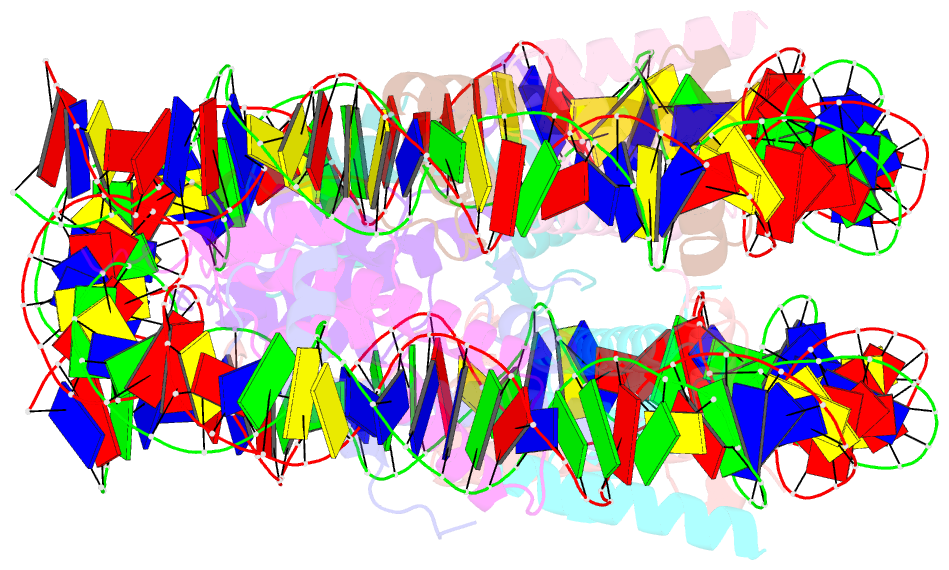
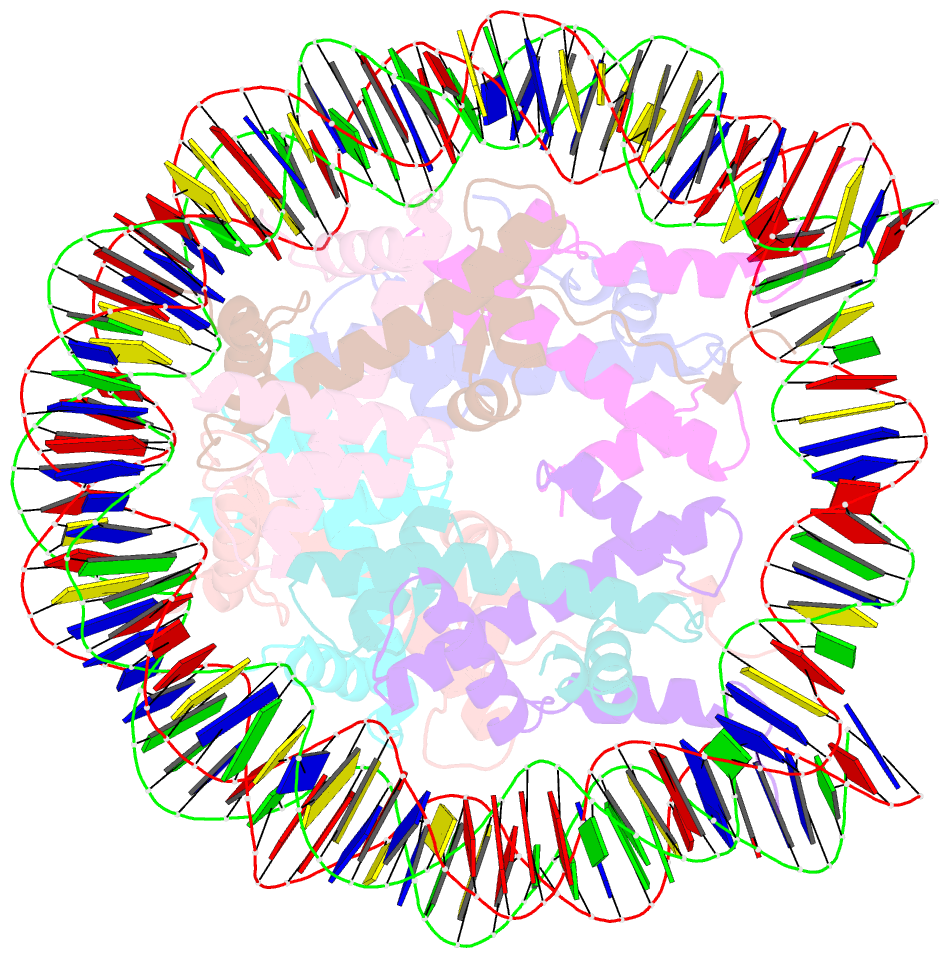
- Crystal structure of the nucleosome containing histone h3 with the crotonylated lysine 122
- Reference
- Suzuki Y, Horikoshi N, Kato D, Kurumizaka H (2016): "Crystal structure of the nucleosome containing histone H3 with crotonylated lysine 122." Biochem.Biophys.Res.Commun., 469, 483-489. doi: 10.1016/j.bbrc.2015.12.041.
- Abstract
- The crotonylation of histones is an important post-translational modification, and epigenetically functions in the regulation of genomic DNA activity. The histone modifications in the structured "histone-fold" domains are considered to have an especially important impact on the nucleosome structure and dynamics. In the present study, we reconstituted the human nucleosome containing histone H3.2 crotonylated at the Lys122 residue, and determined its crystal structure at 2.56 Å resolution. We found that the crotonylation of the H3 Lys122 residue does not affect the overall nucleosome structure, but locally impedes the formation of the water-mediated hydrogen bond with the DNA backbone. Consistently, thermal stability assays revealed that the H3 Lys122 crotonylation, as well as the H3 Lys122 acetylation, clearly reduced the histone-DNA association.