Summary information and primary citation
- PDB-id
- 5co8; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (2.4 Å)
- Summary
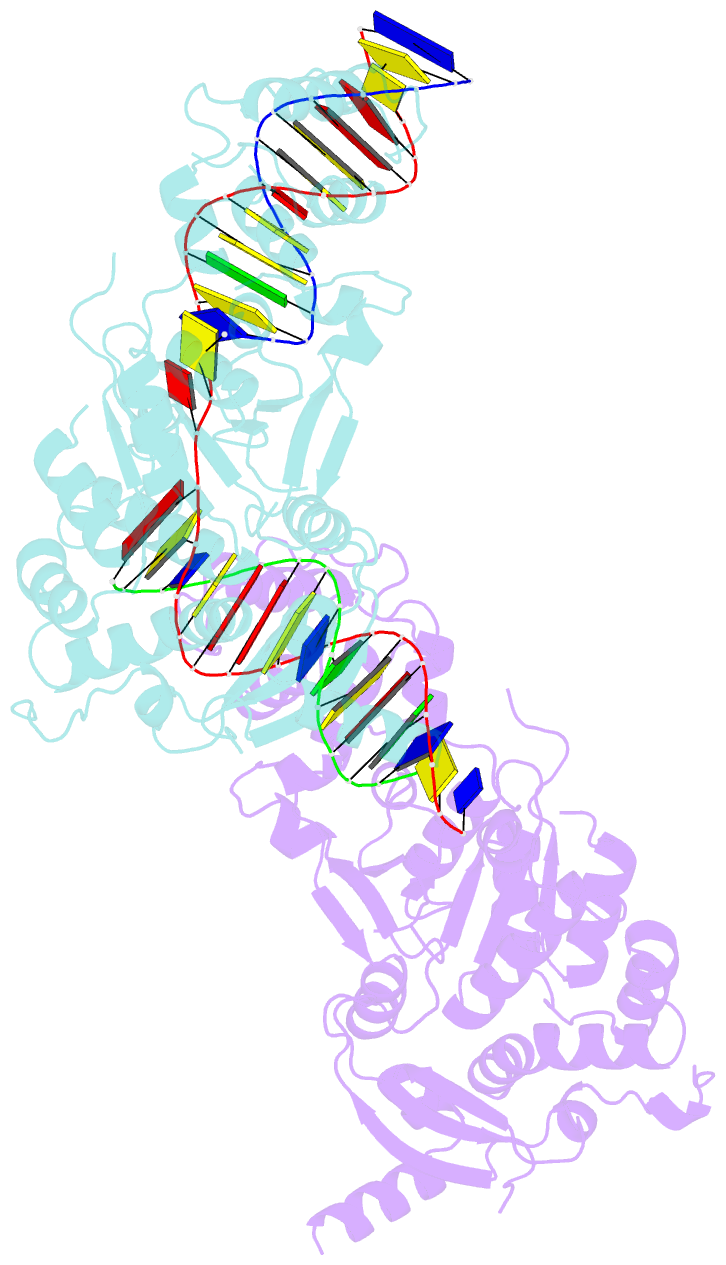
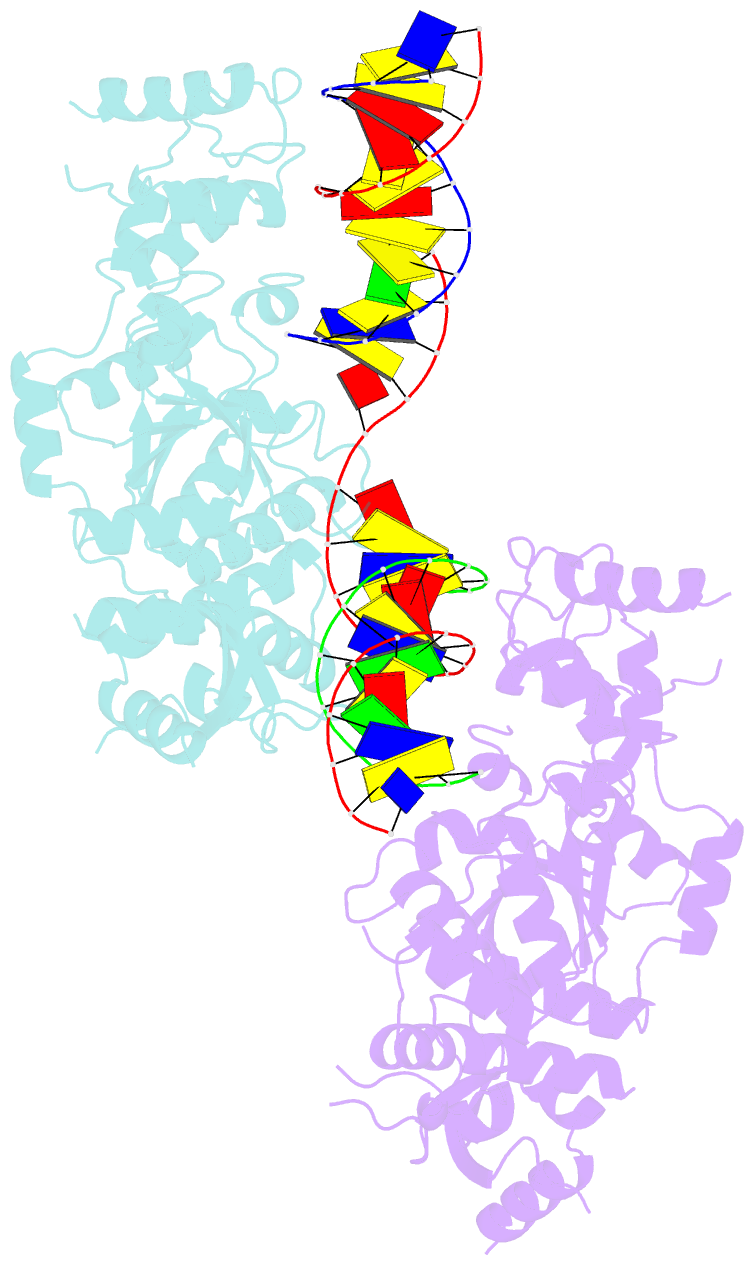
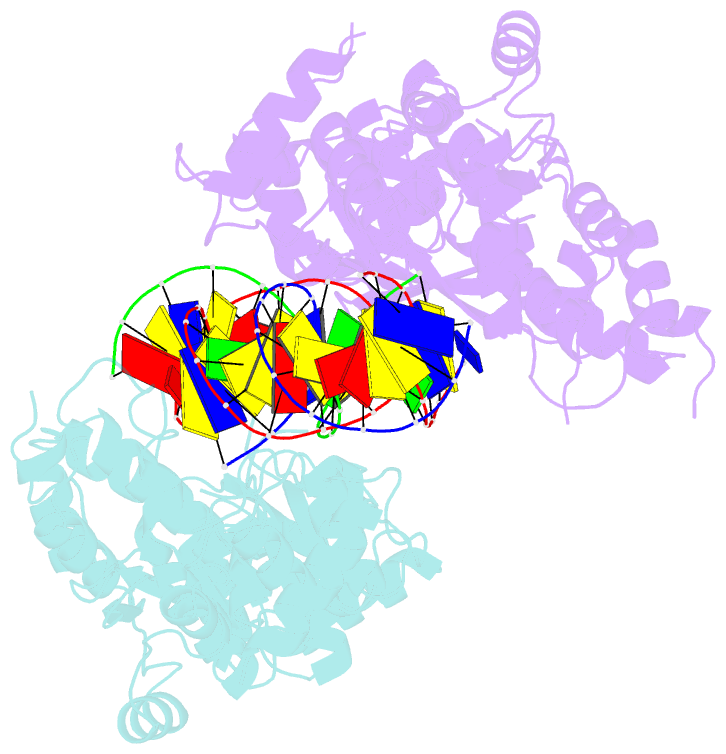
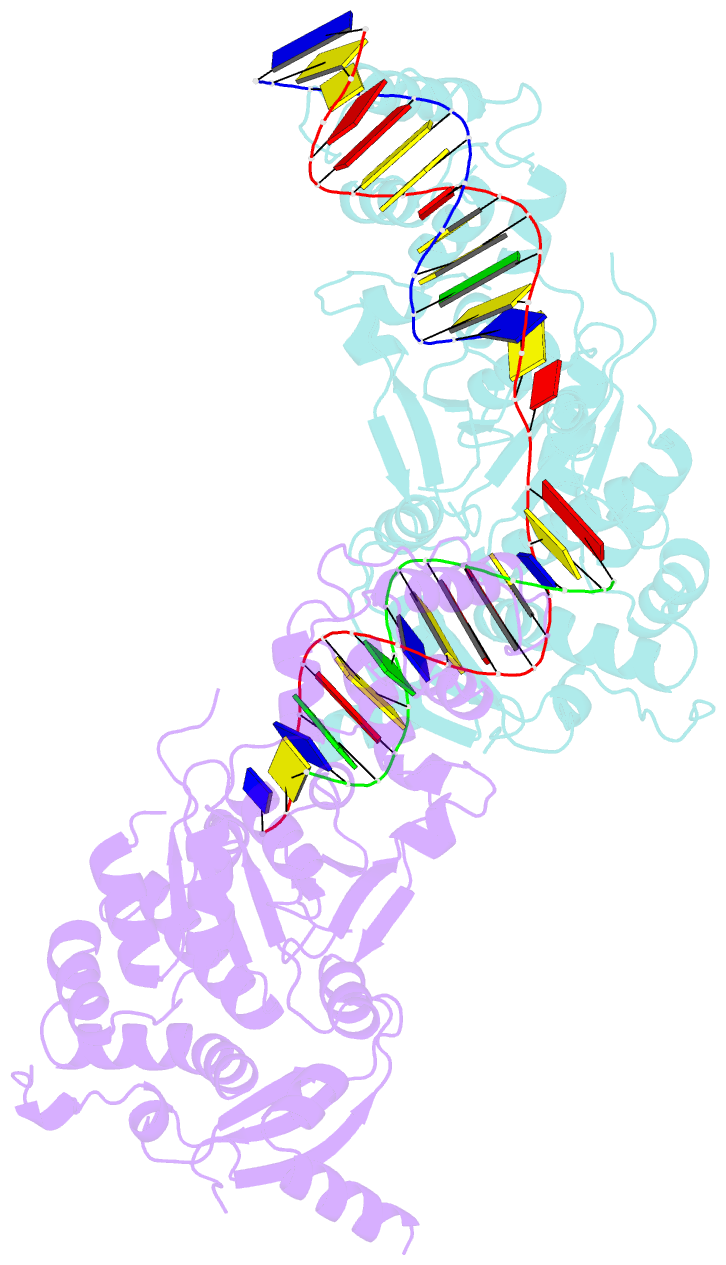
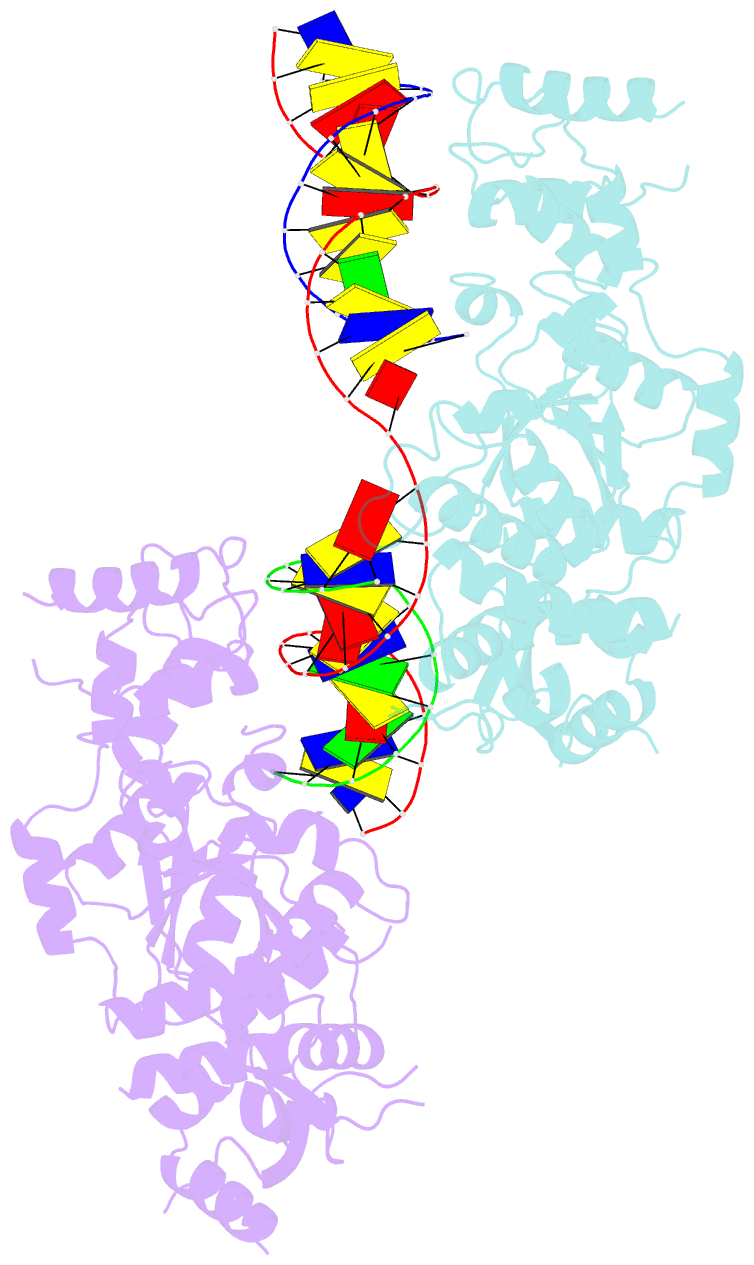
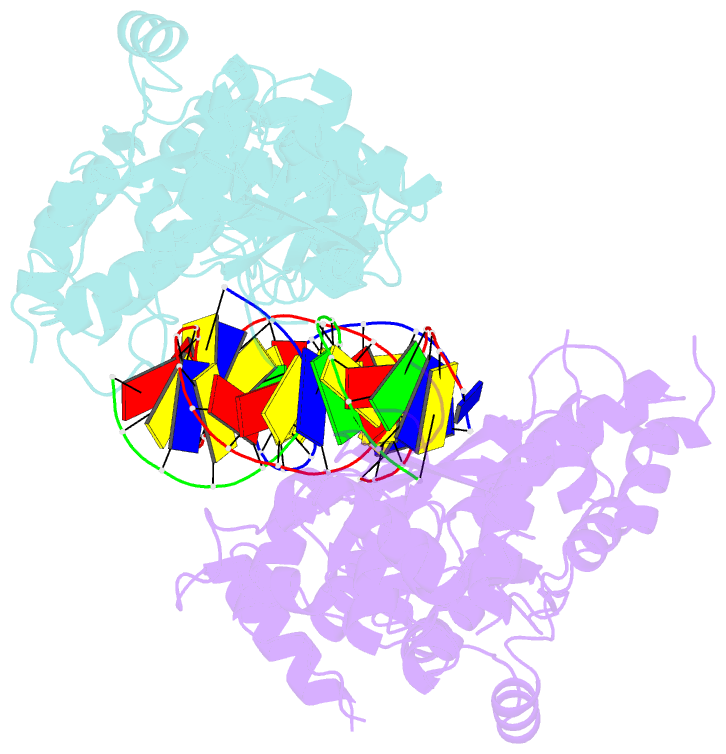
- Crystal structure of the holliday junction-resolving enzyme gen1 (wt) in complex with product DNA and mg2+ ion
- Reference
- Liu Y, Freeman AD, Declais AC, Wilson TJ, Gartner A, Lilley DM (2015): "Crystal Structure of a Eukaryotic GEN1 Resolving Enzyme Bound to DNA." Cell Rep, 13, 2565-2575. doi: 10.1016/j.celrep.2015.11.042.
- Abstract
- We present the crystal structure of the junction-resolving enzyme GEN1 bound to DNA at 2.5 Å resolution. The structure of the GEN1 protein reveals it to have an elaborated FEN-XPG family fold that is modified for its role in four-way junction resolution. The functional unit in the crystal is a monomer of active GEN1 bound to the product of resolution cleavage, with an extensive DNA binding interface for both helical arms. Within the crystal lattice, a GEN1 dimer interface juxtaposes two products, whereby they can be reconnected into a four-way junction, the structure of which agrees with that determined in solution. The reconnection requires some opening of the DNA structure at the center, in agreement with permanganate probing and 2-aminopurine fluorescence. The structure shows that a relaxation of the DNA structure accompanies cleavage, suggesting how second-strand cleavage is accelerated to ensure productive resolution of the junction.