Summary information and primary citation
- PDB-id
- 5d3g; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transferase
- Method
- X-ray (2.3 Å)
- Summary
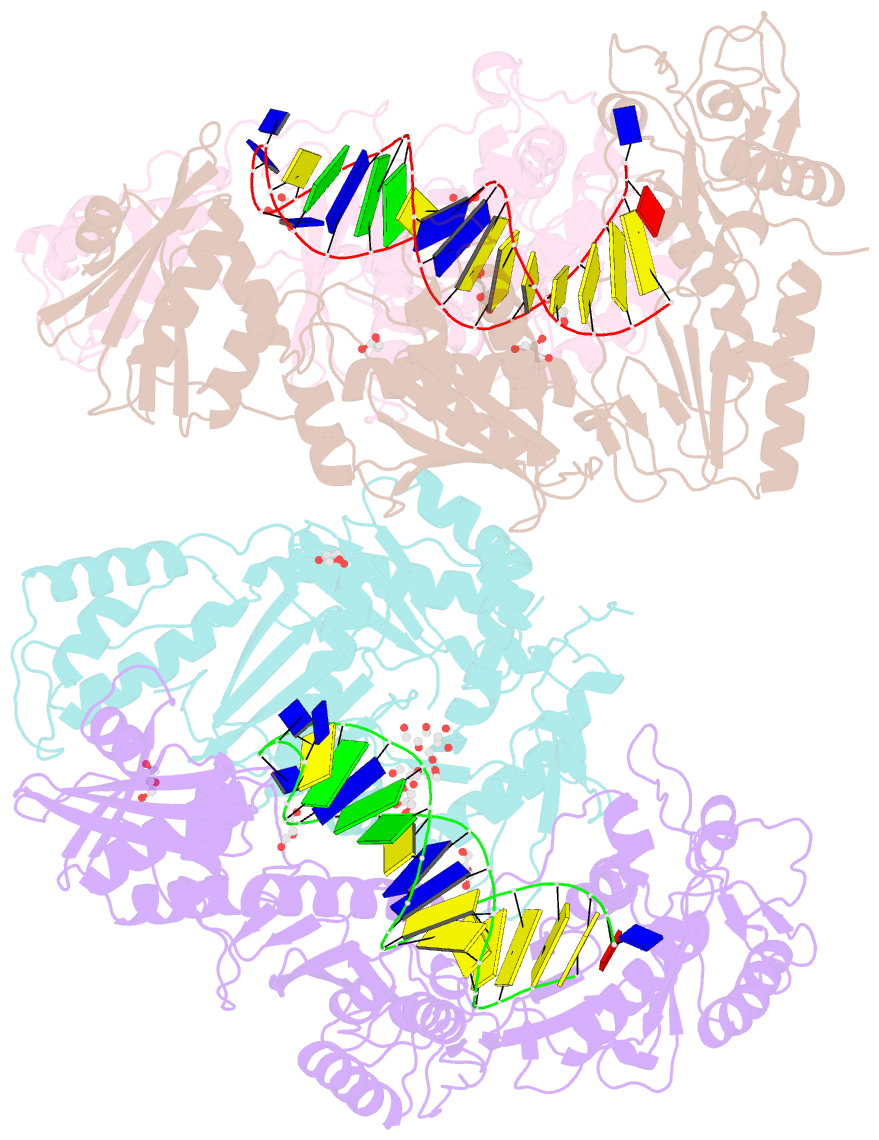
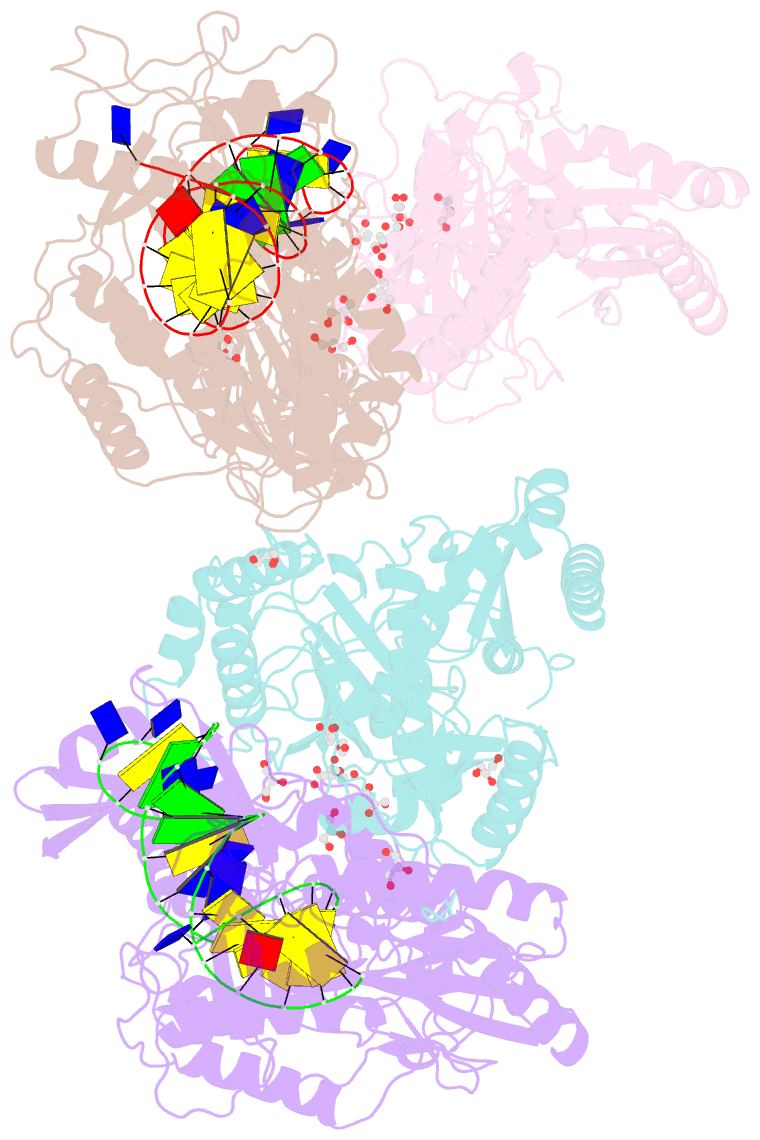
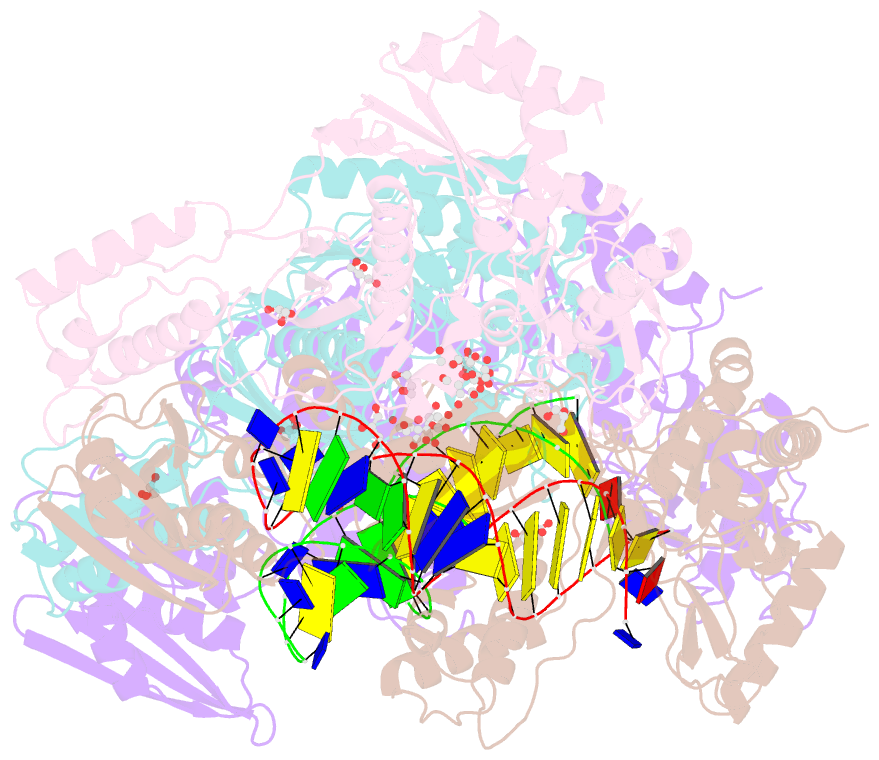
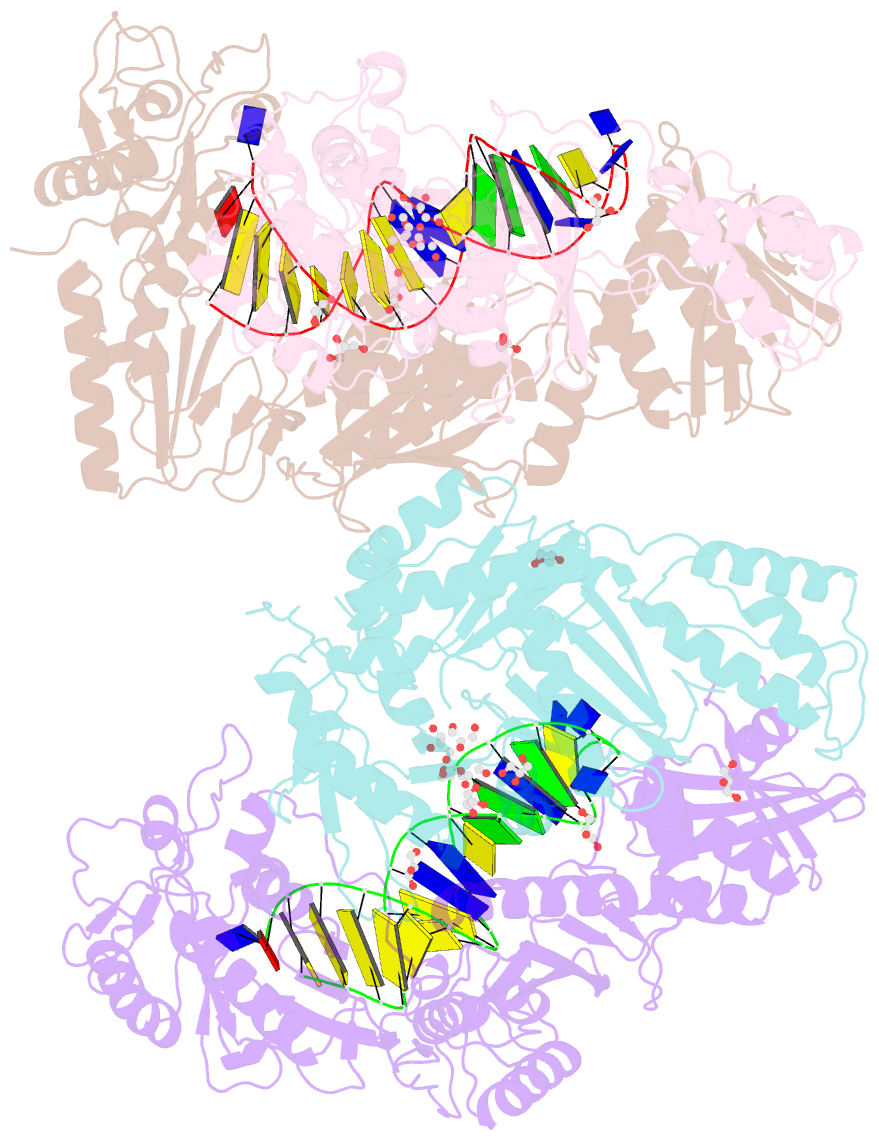
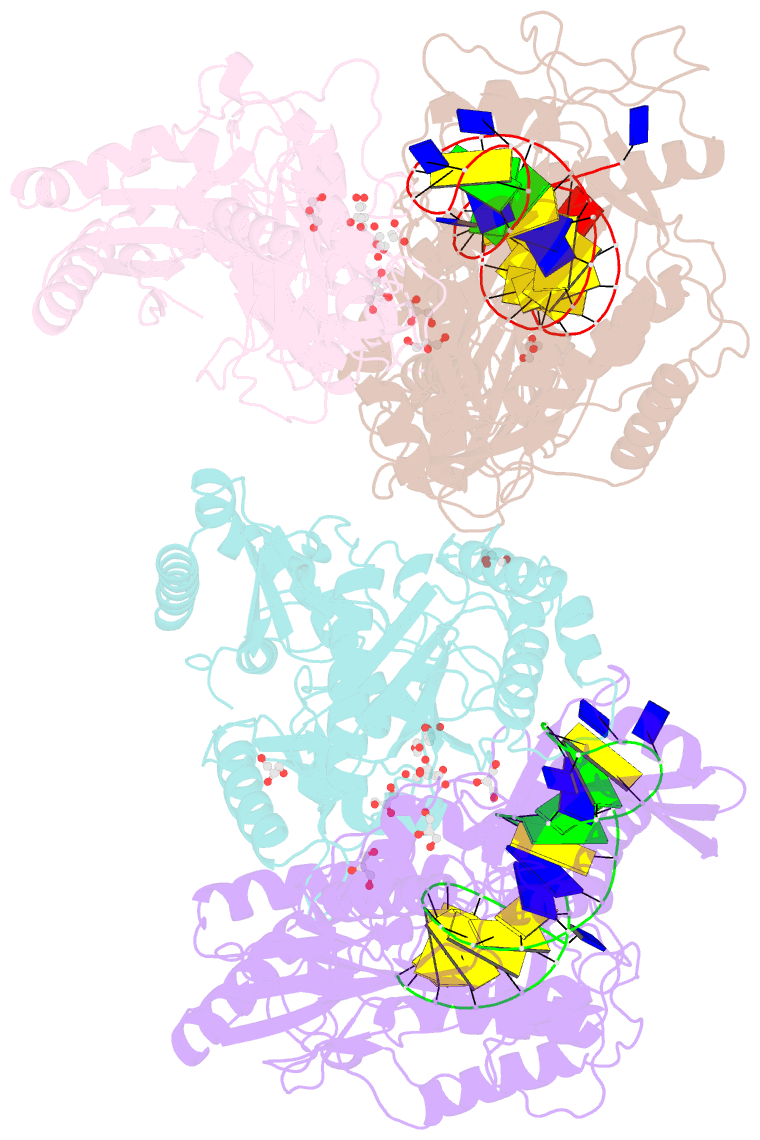
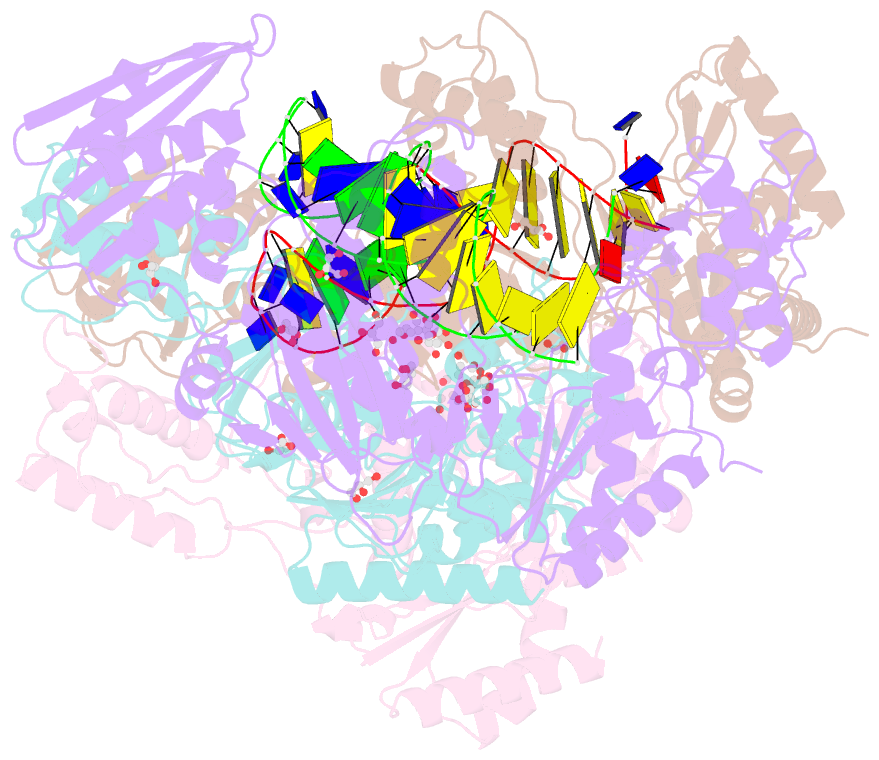
- Structure of hiv-1 reverse transcriptase bound to a novel 38-mer hairpin template-primer DNA aptamer
- Reference
- Miller MT, Tuske S, Das K, DeStefano JJ, Arnold E (2016): "Structure of HIV-1 reverse transcriptase bound to a novel 38-mer hairpin template-primer DNA aptamer." Protein Sci., 25, 46-55. doi: 10.1002/pro.2776.
- Abstract
- The development of a modified DNA aptamer that binds HIV-1 reverse transcriptase (RT) with ultra-high affinity has enabled the X-ray structure determination of an HIV-1 RT-DNA complex to 2.3 Å resolution without the need for an antibody Fab fragment or RT-DNA cross-linking. The 38-mer hairpin-DNA aptamer has a 15 base-pair duplex, a three-deoxythymidine hairpin loop, and a five-nucleotide 5'-overhang. The aptamer binds RT in a template-primer configuration with the 3'-end positioned at the polymerase active site and has 2'-O-methyl modifications at the second and fourth duplex template nucleotides that interact with the p66 fingers and palm subdomains. This structure represents the highest resolution RT-nucleic acid structure to date. The RT-aptamer complex is catalytically active and can serve as a platform for studying fundamental RT mechanisms and for development of anti-HIV inhibitors through fragment screening and other approaches. Additionally, the structure allows for a detailed look at a unique aptamer design and provides the molecular basis for its remarkably high affinity for RT.