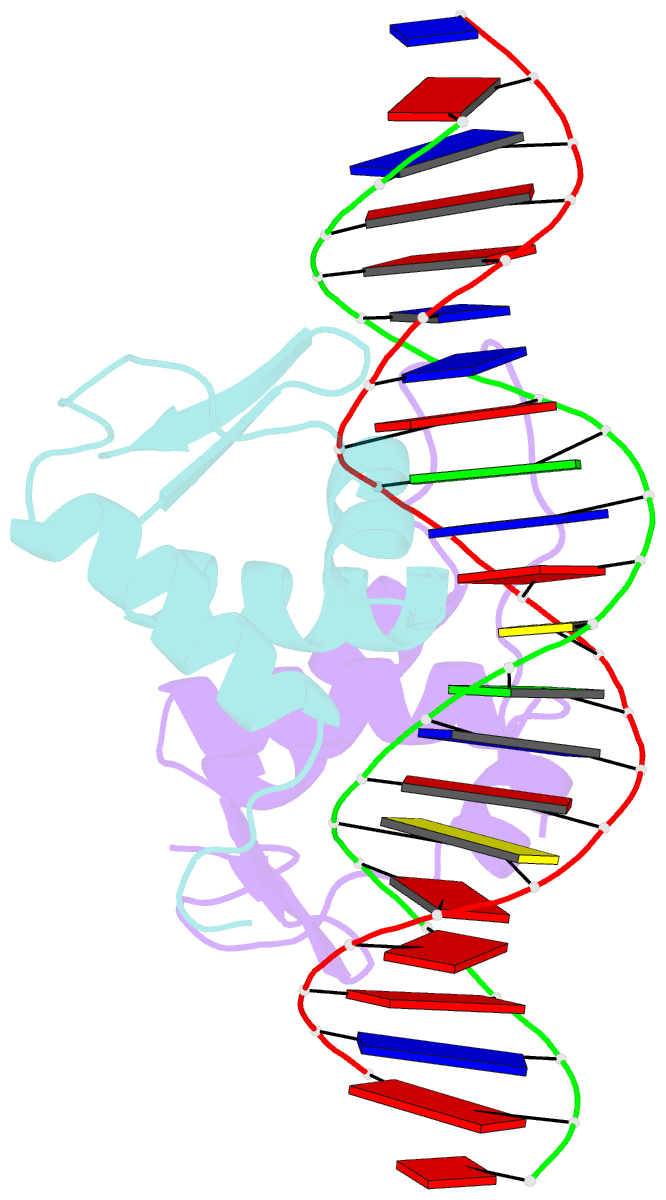
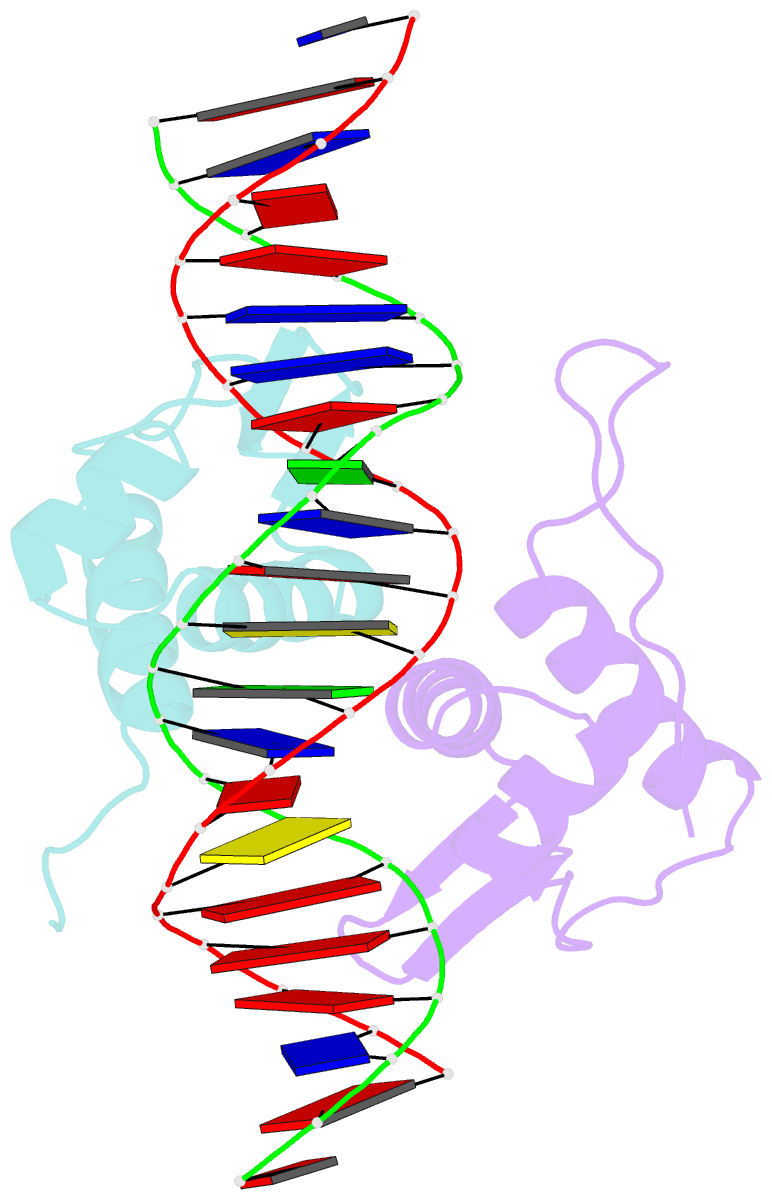
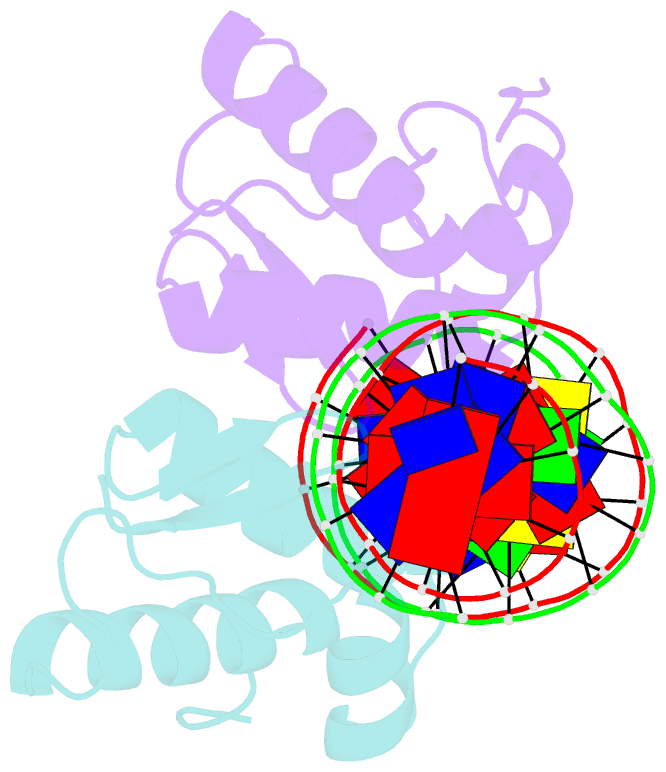
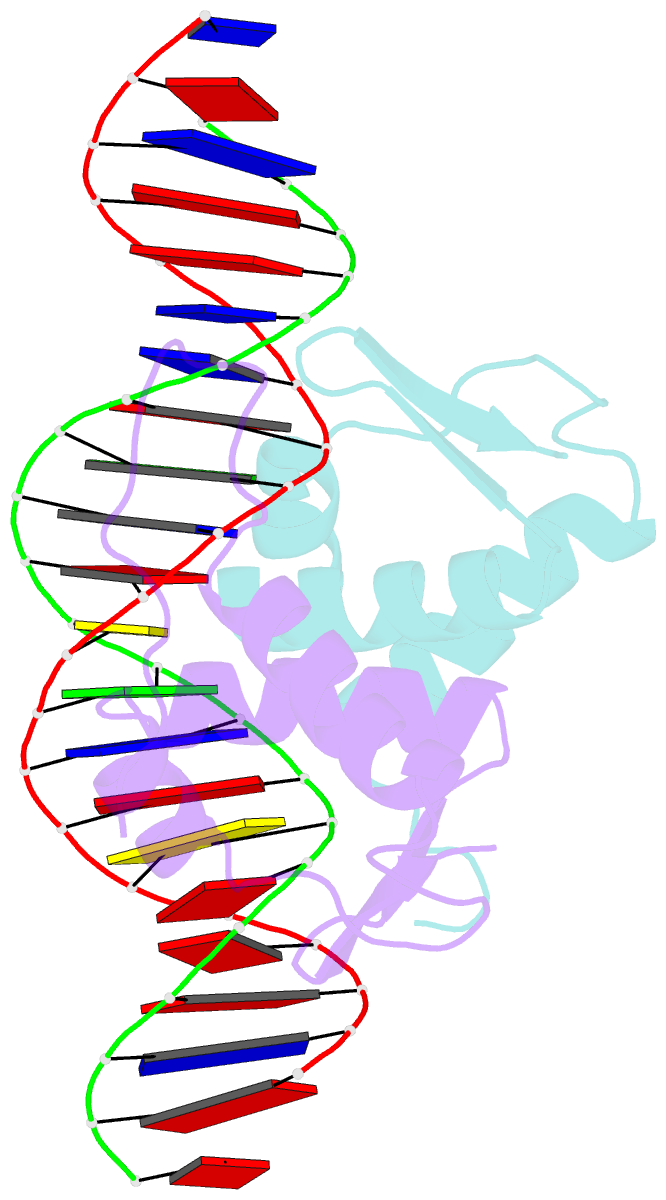
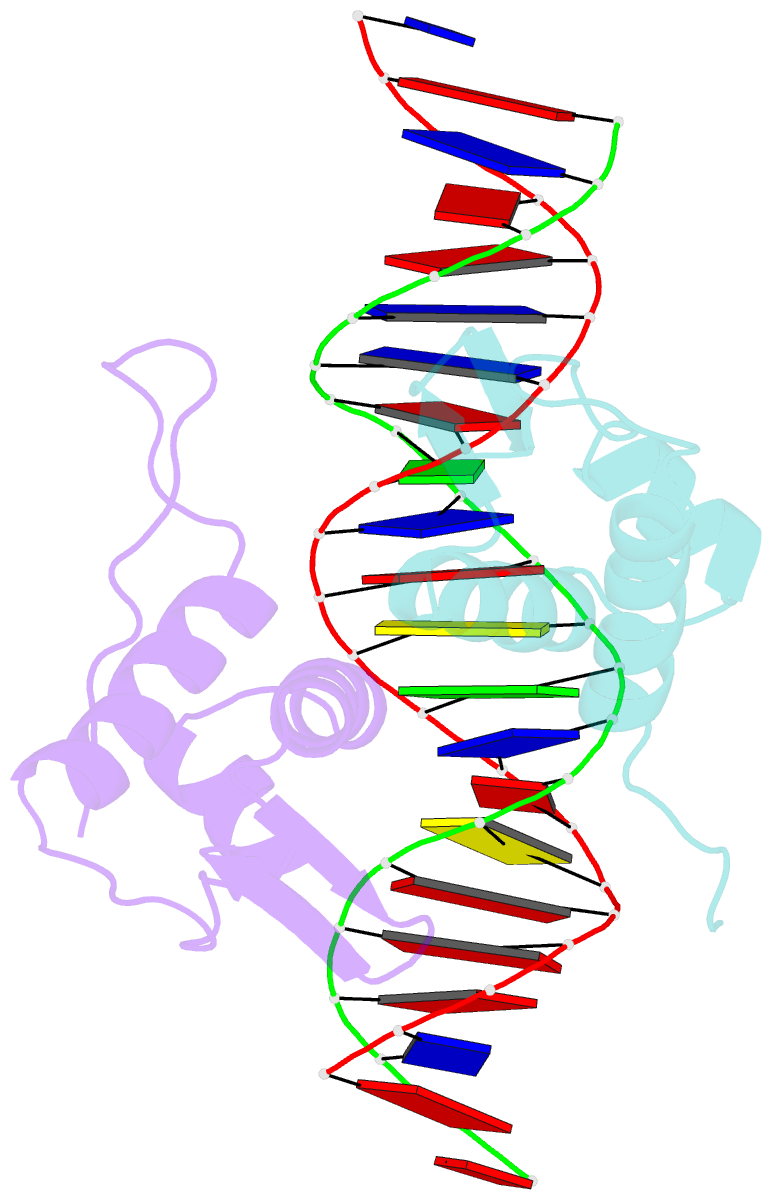
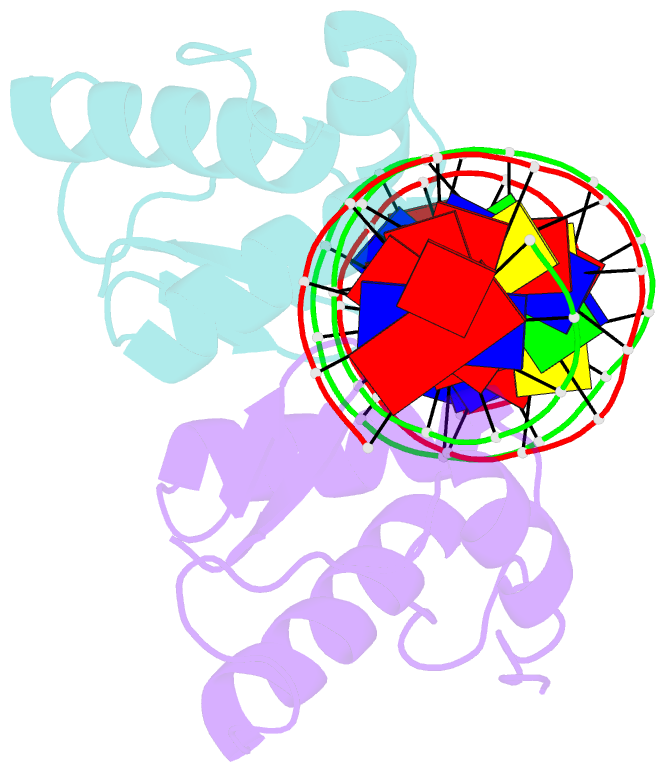
Summary information and primary citation
- PDB-id
- 5d4r; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.07 Å)
- Summary
- Crystal structure of arar(dbd) in complex with operator ore1
- Reference
- Jain D, Narayanan N, Nair DT (2016): "Plasticity in Repressor-DNA Interactions Neutralizes Loss of Symmetry in Bipartite Operators." J.Biol.Chem., 291, 1235-1242. doi: 10.1074/jbc.M115.689695.
- Abstract
- Transcription factor-DNA interactions are central to gene regulation. Many transcription factors regulate multiple target genes and can bind sequences that do not conform strictly to the consensus. To understand the structural mechanism utilized by the transcription regulators to bind diverse target sequences, we have employed the repressor AraR from Bacillus subtilis as a model system. AraR is known to bind to eight different operator sites in the bacterial genome. Although there are differences in the sequences of four of these operators, ORE1, ORX1, ORA1, and ORR3, the AraR-DNA binding domain (AraR-DBD) as well as full-length AraR unexpectedly binds to each of these sequences with similar affinities as measured by fluorescence anisotropy experiments. We have determined crystal structures of AraR-DBD in complex with two different natural operators ORE1 and ORX1 up to 2.07 and 1.97 Å resolution, respectively. These structures were compared with the previously reported structures of AraR-DBD bound to two other natural operators (ORA1 and ORR3). Interactions of two molecules of AraR-DBD with the symmetric operator, ORE1, are identical, but their interaction with the non-symmetric operator ORX1 results in breakdown of the symmetry in protein-DNA interactions. The novel interactions observed are accompanied by local conformational change in the DNA. ChIP-sequencing (ChIP-Seq) data on other transcription factors has shown that they can bind to diverse targets, and hence the plasticity exhibited by AraR may be a general phenomenon. The ability of transcription factors to form alternate interactions may be important for employment in new functions and evolution of novel regulatory circuits.