Summary information and primary citation
- PDB-id
- 5dac; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (2.503 Å)
- Summary
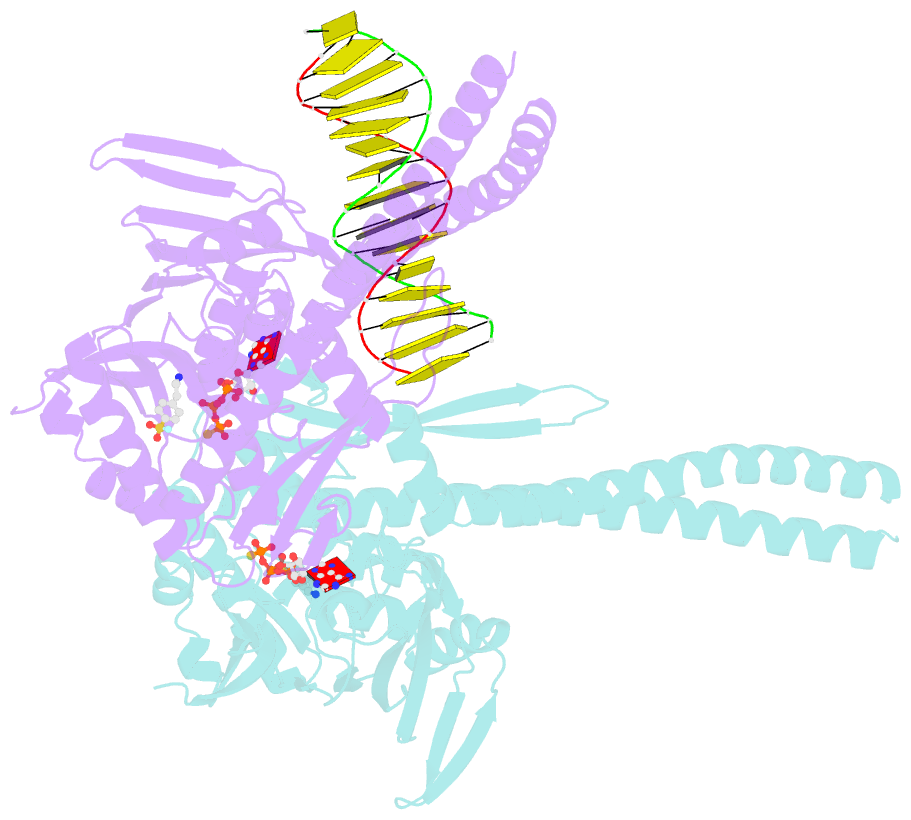
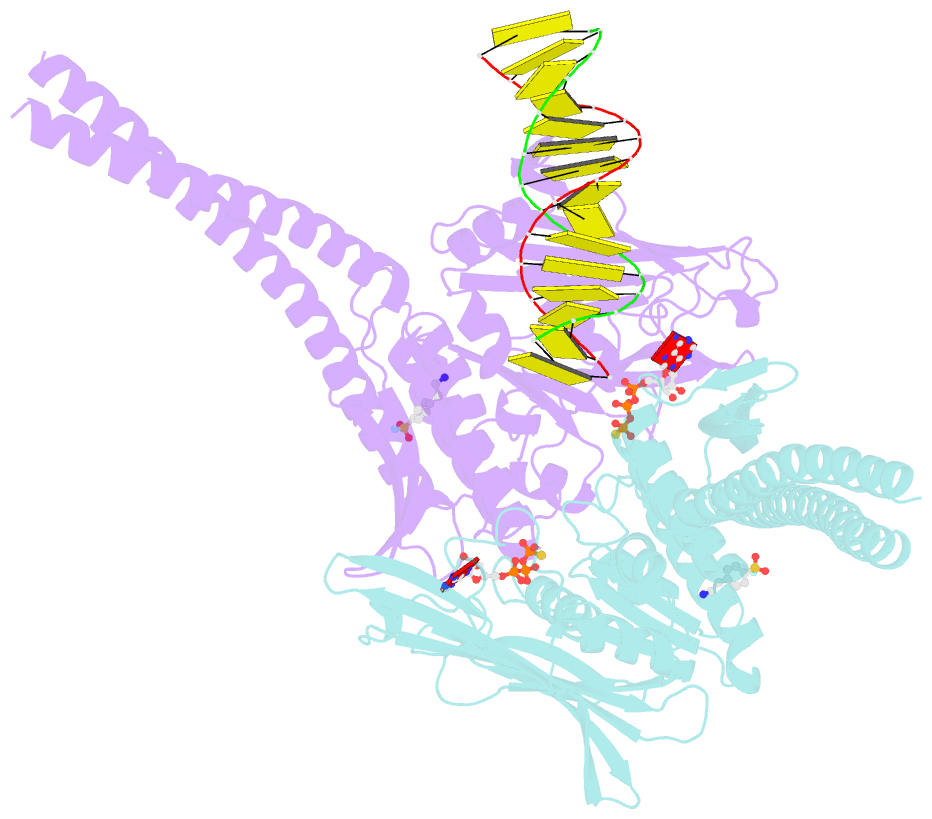
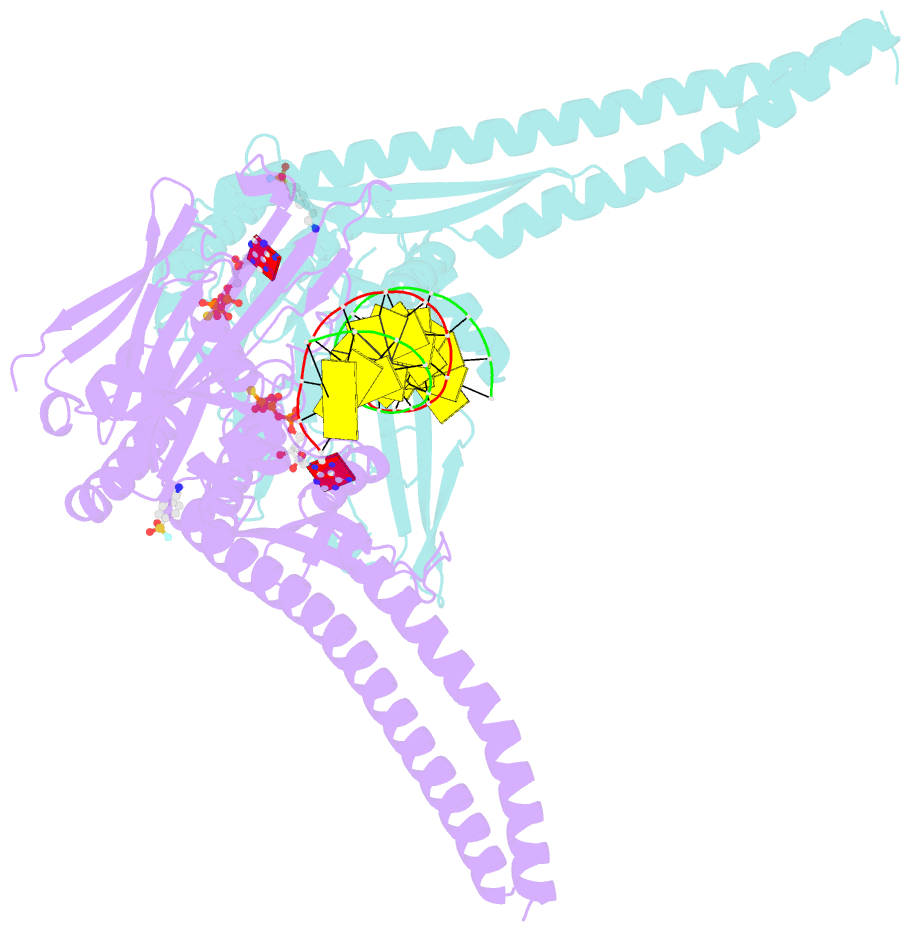
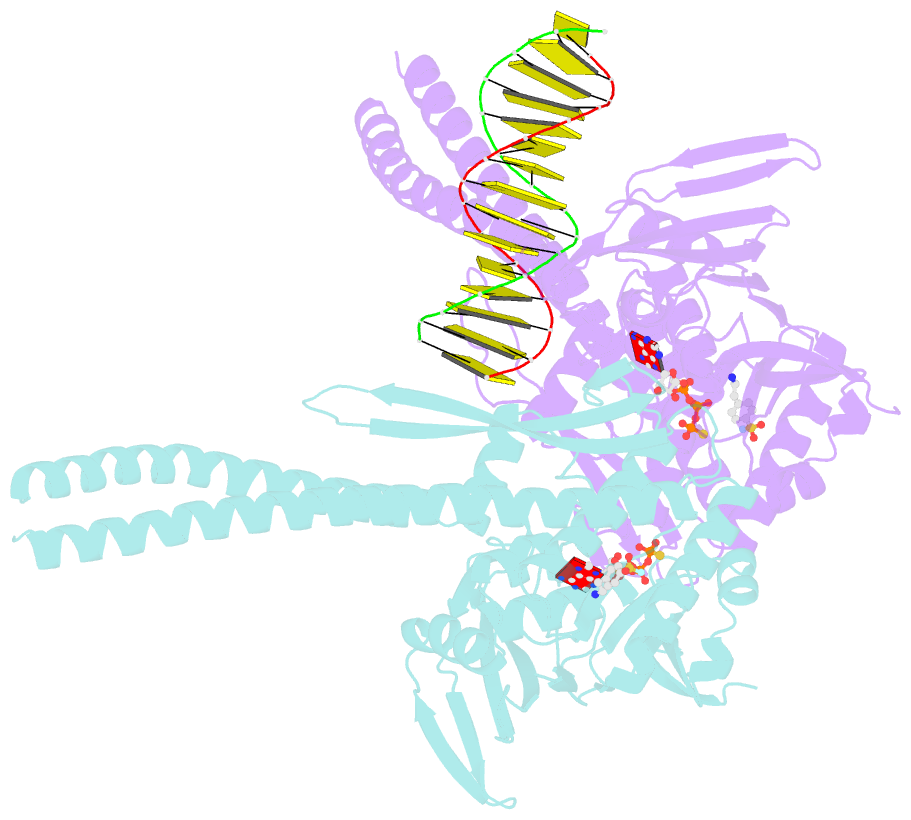
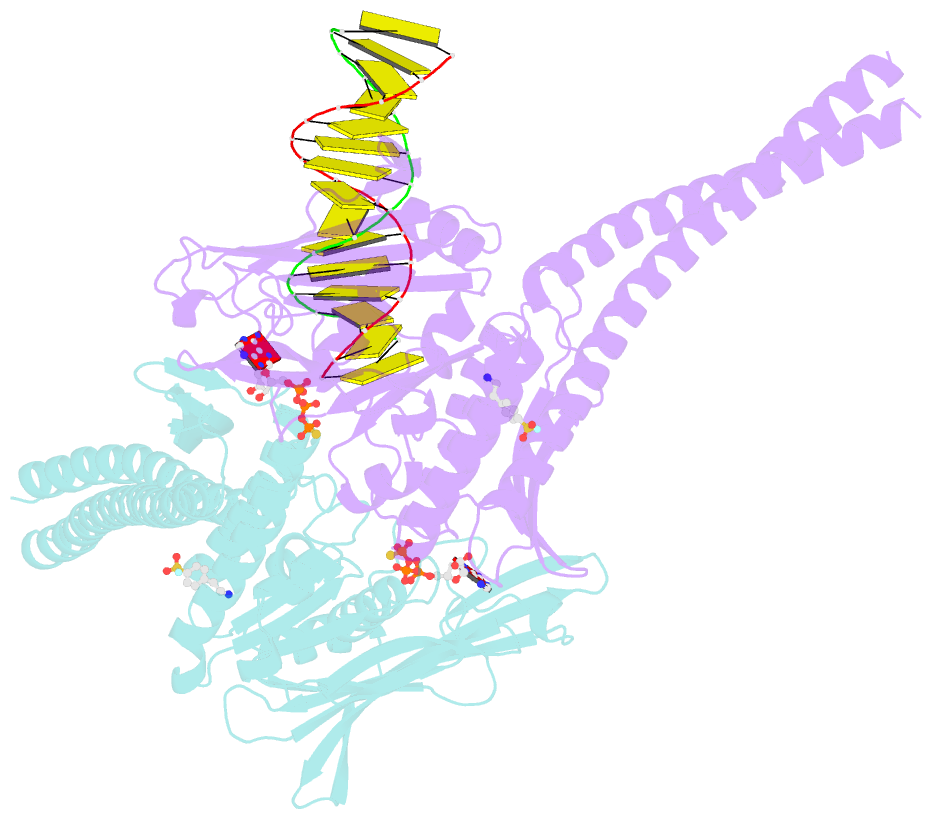
- Atp-gamma-s bound rad50 from chaetomium thermophilum in complex with DNA
- Reference
- Seifert FU, Lammens K, Stoehr G, Kessler B, Hopfner KP (2016): "Structural mechanism of ATP-dependent DNA binding and DNA end bridging by eukaryotic Rad50." Embo J., 35, 759-772. doi: 10.15252/embj.201592934.
- Abstract
- The Mre11-Rad50-Nbs1 (MRN) complex is a central factor in the repair of DNA double-strand breaks (DSBs). The ATP-dependent mechanisms of how MRN detects and endonucleolytically processes DNA ends for the repair by microhomology-mediated end-joining or further resection in homologous recombination are still unclear. Here, we report the crystal structures of the ATPγS-bound dimer of the Rad50(NBD)(nucleotide-binding domain) from the thermophilic eukaryote Chaetomium thermophilum(Ct) in complex with either DNA or CtMre11(RBD)(Rad50-binding domain) along with small-angle X-ray scattering and cross-linking studies. The structure and DNA binding motifs were validated by DNA binding experiments in vitro and mutational analyses in Saccharomyces cerevisiae in vivo Our analyses provide a structural framework for the architecture of the eukaryotic Mre11-Rad50 complex. They show that a Rad50 dimer binds approximately 18 base pairs of DNA along the dimer interface in anATP-dependent fashion or bridges two DNA ends with a preference for 3' overhangs. Finally, our results may provide a general framework for the interaction of ABC ATPase domains of the Rad50/SMC/RecN protein family with DNA.