Summary information and primary citation
- PDB-id
- 5dny; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- recombination-DNA
- Method
- X-ray (3.11 Å)
- Summary
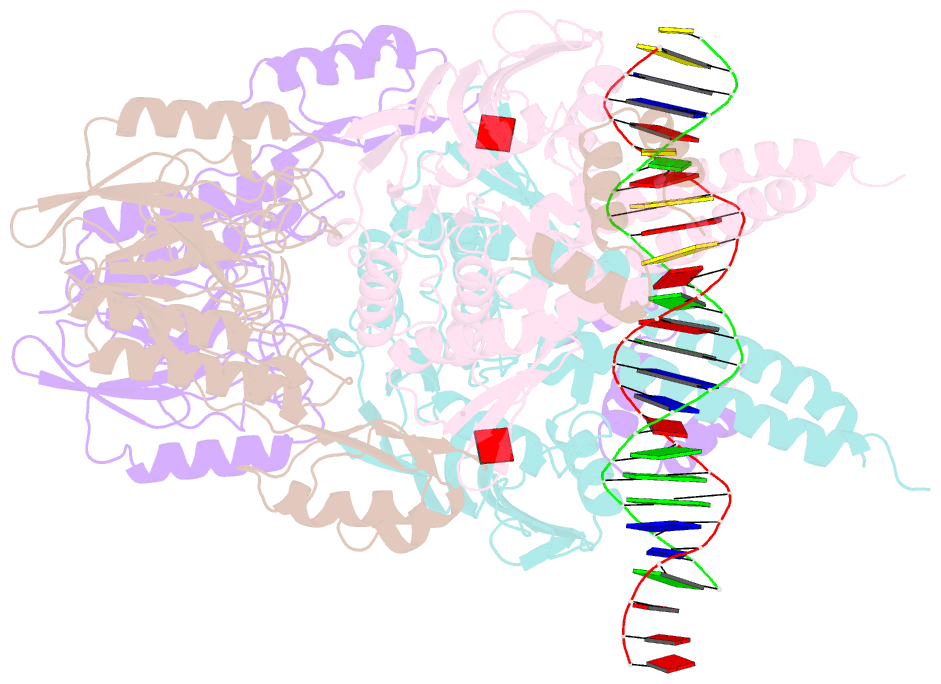
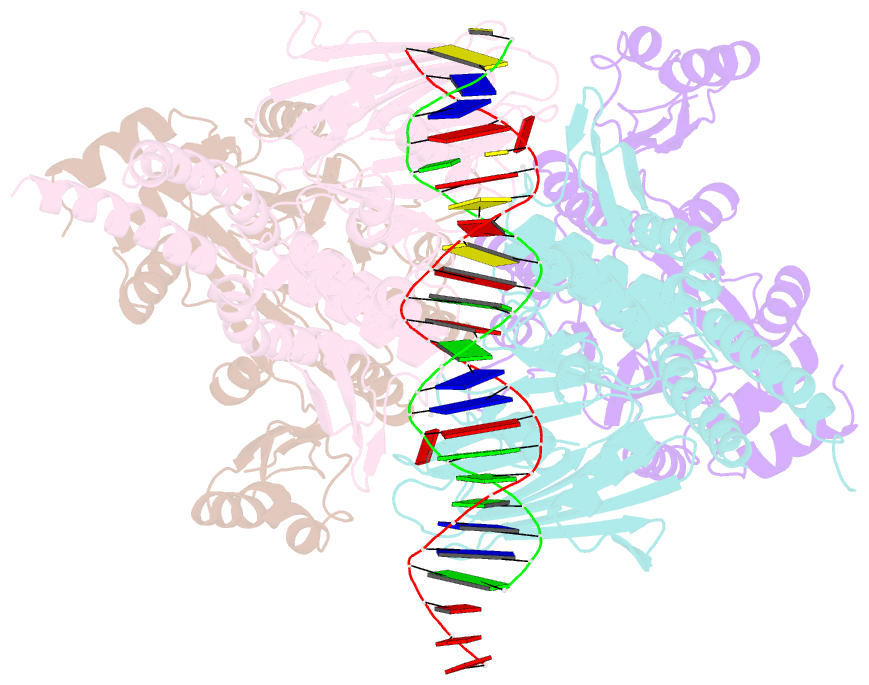
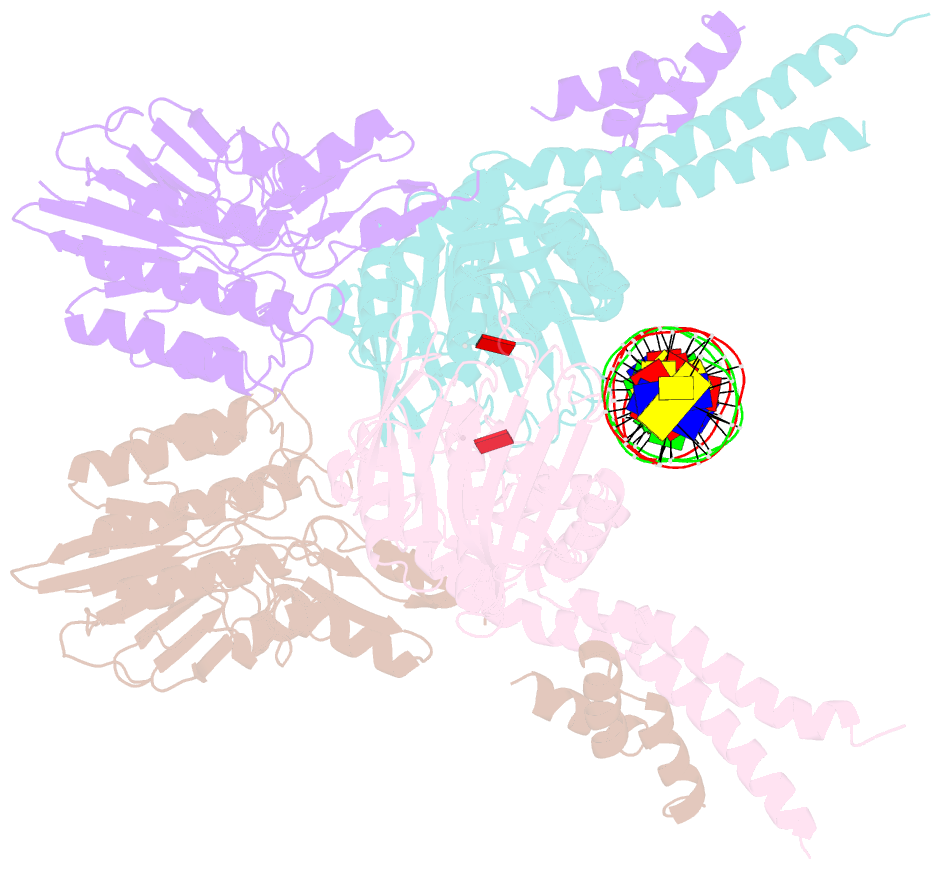
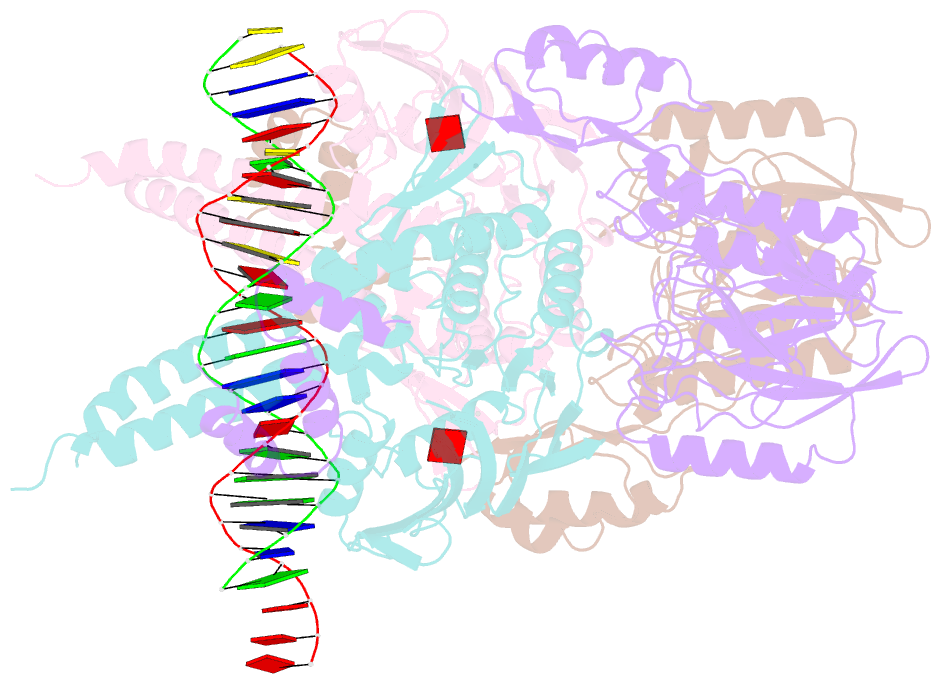
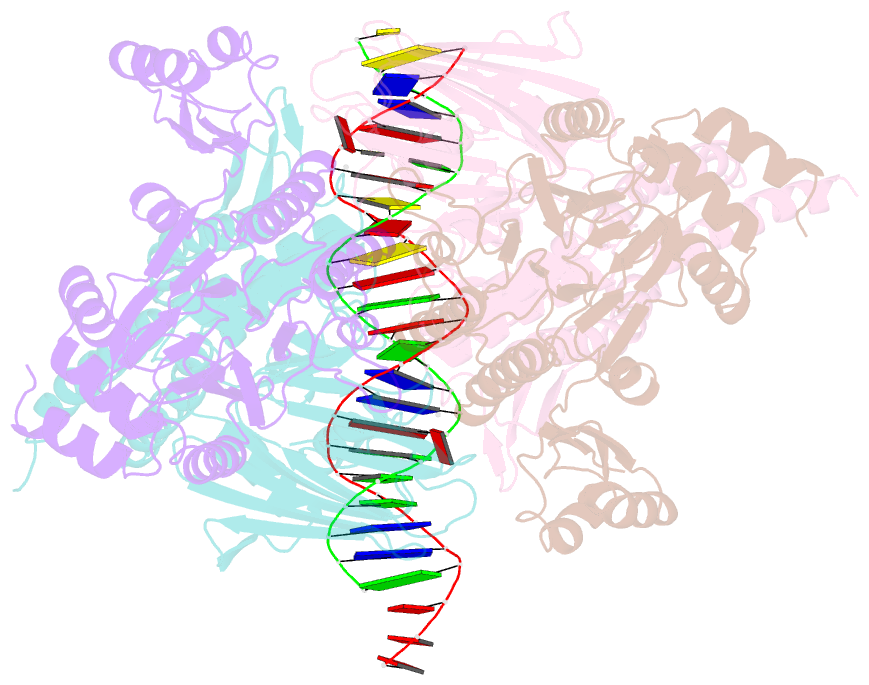
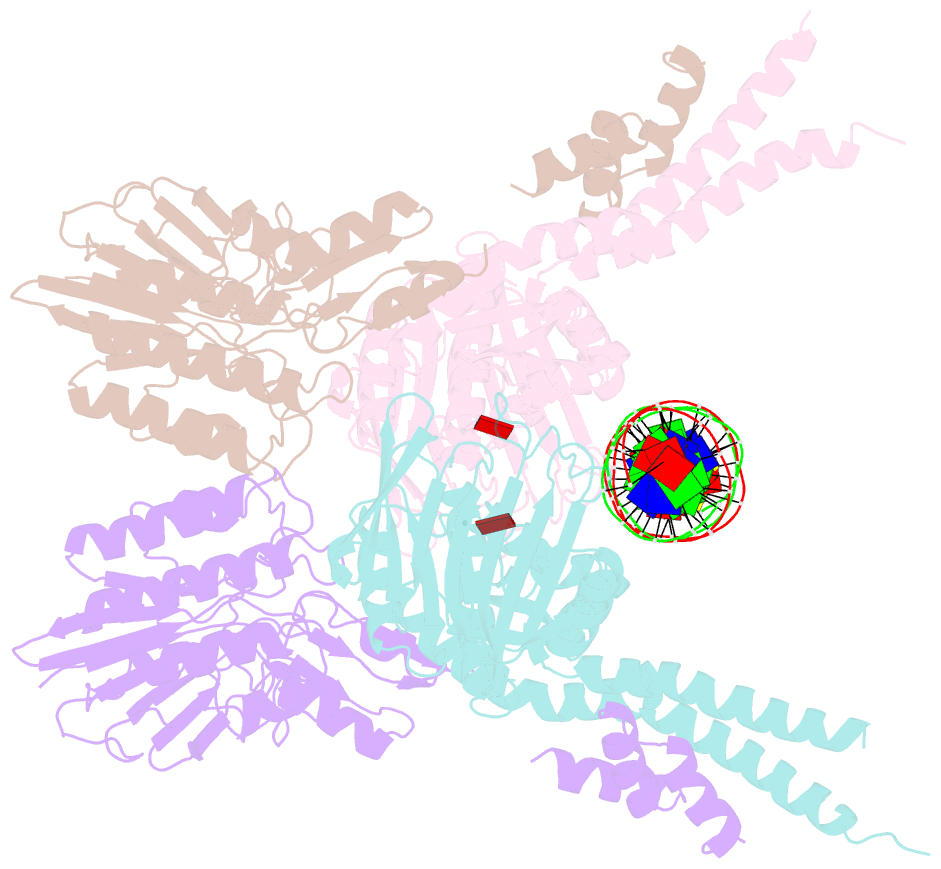
- Structure of the atprs-mre11-rad50-DNA complex
- Reference
- Liu Y, Sung S, Kim Y, Li F, Gwon G, Jo A, Kim AK, Kim T, Song OK, Lee SE, Cho Y (2016): "ATP-dependent DNA binding, unwinding, and resection by the Mre11/Rad50 complex." Embo J., 35, 743-758. doi: 10.15252/embj.201592462.
- Abstract
- ATP-dependent DNA end recognition and nucleolytic processing are central functions of the Mre11/Rad50 (MR) complex in DNA double-strand break repair. However, it is still unclear how ATP binding and hydrolysis primes the MR function and regulates repair pathway choice in cells. Here,Methanococcus jannaschii MR-ATPγS-DNA structure reveals that the partly deformed DNA runs symmetrically across central groove between two ATPγS-bound Rad50 nucleotide-binding domains. Duplex DNA cannot access the Mre11 active site in the ATP-free full-length MR complex. ATP hydrolysis drives rotation of the nucleotide-binding domain and induces the DNA melting so that the substrate DNA can access Mre11. Our findings suggest that the ATP hydrolysis-driven conformational changes in both DNA and the MR complex coordinate the melting and endonuclease activity.