Summary information and primary citation
- PDB-id
- 5dqz; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (2.7 Å)
- Summary
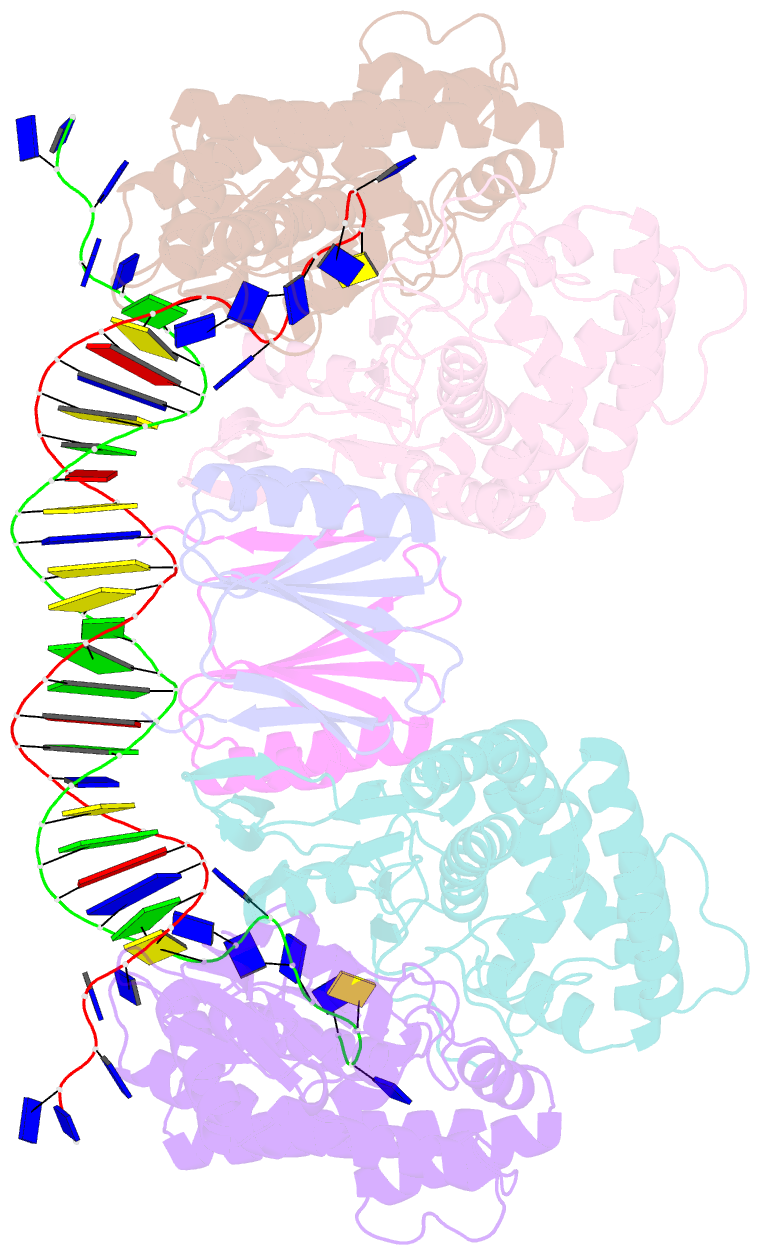
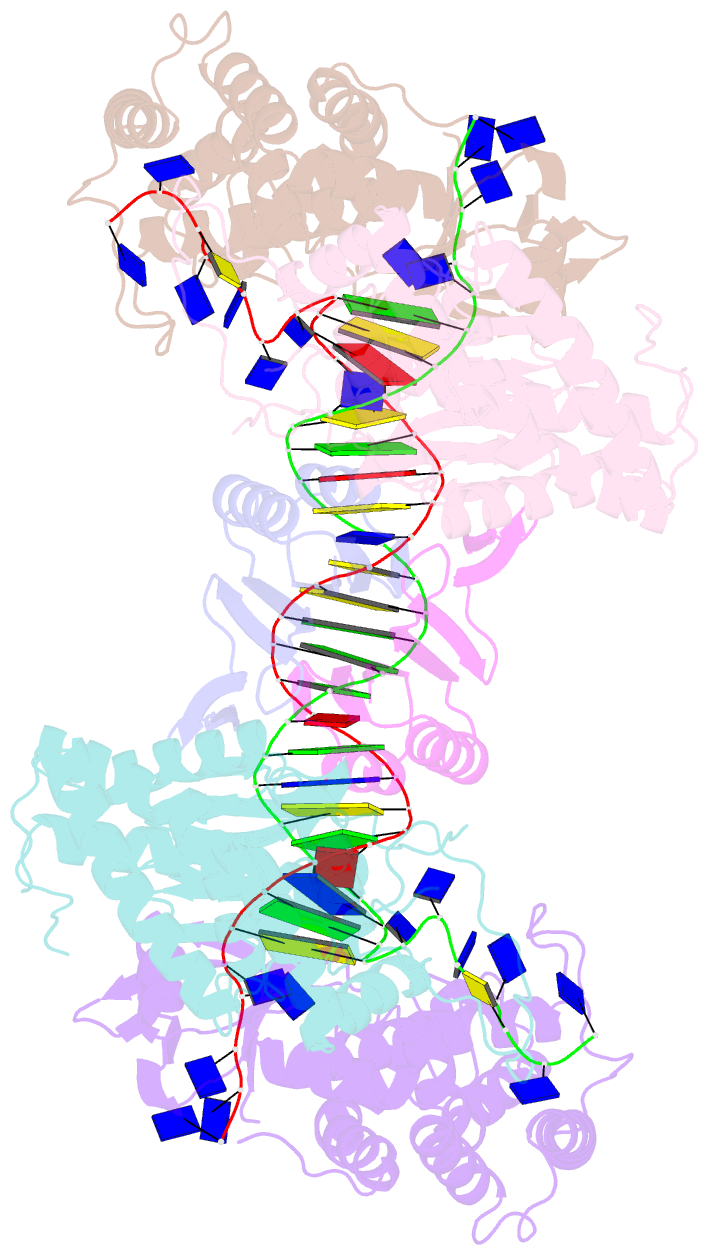
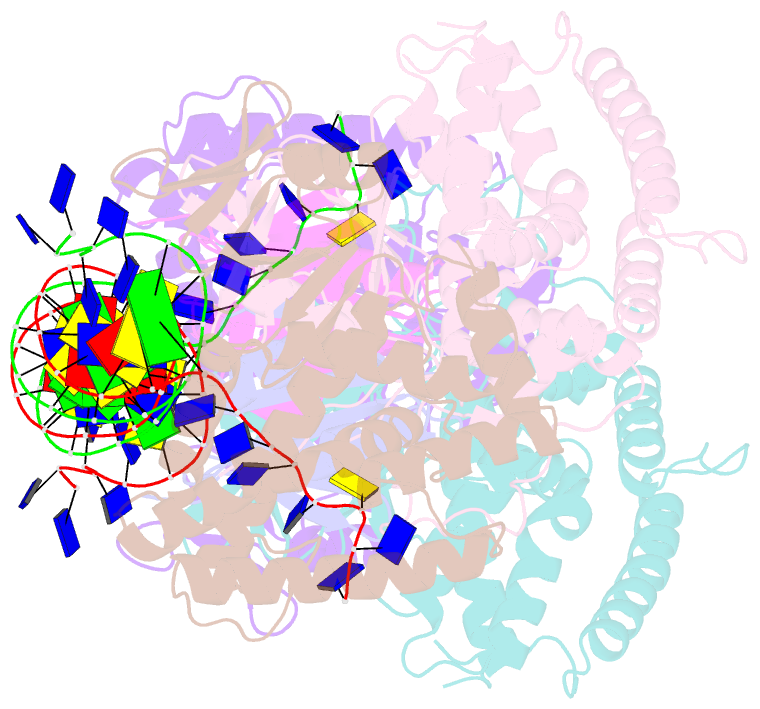
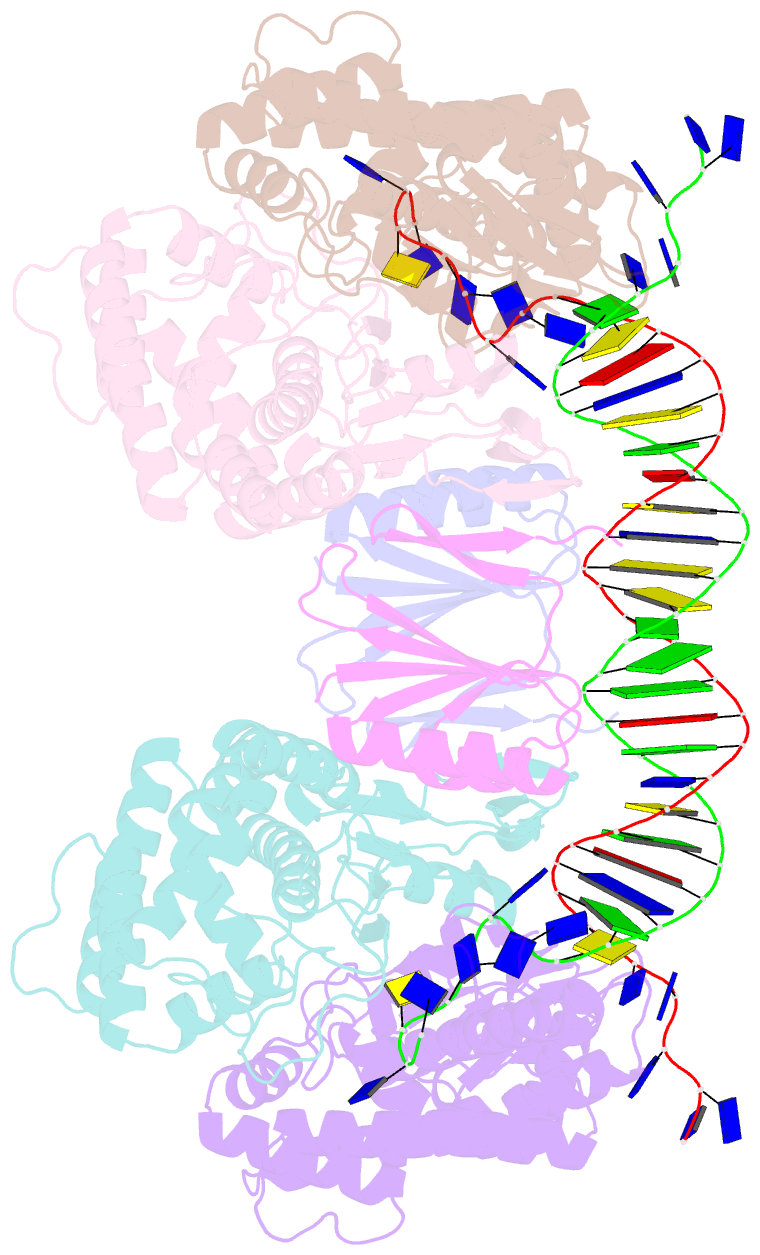
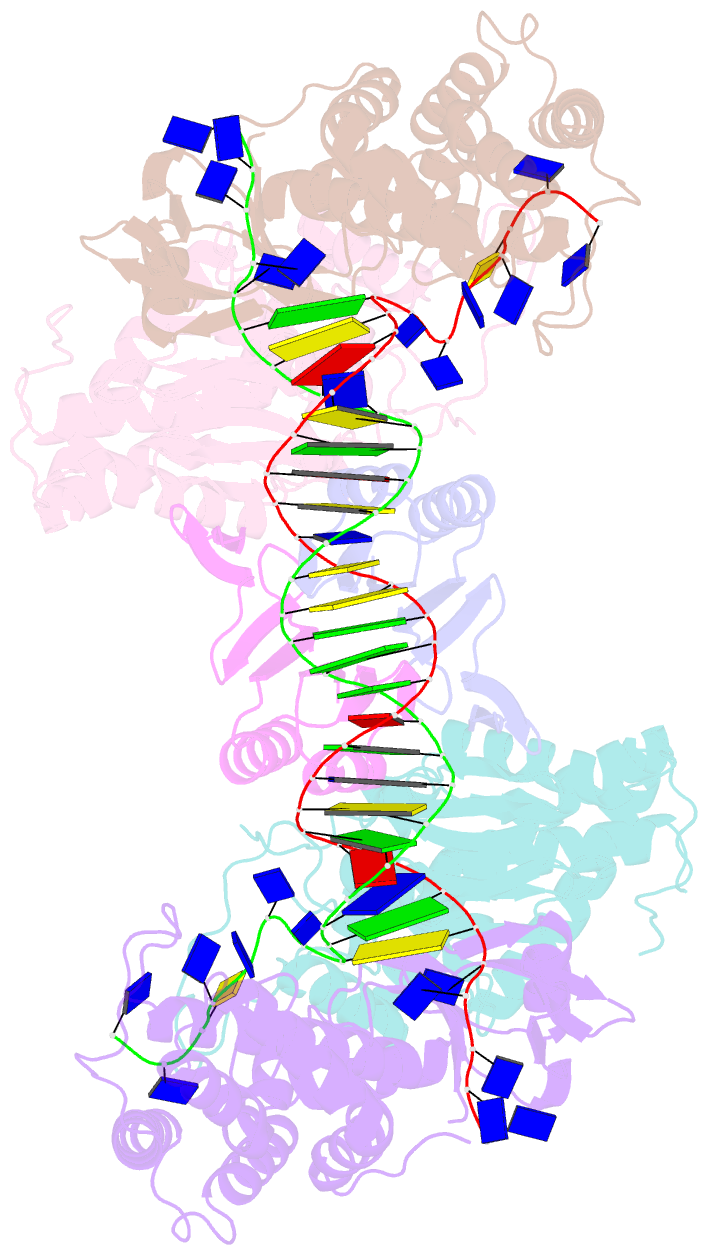
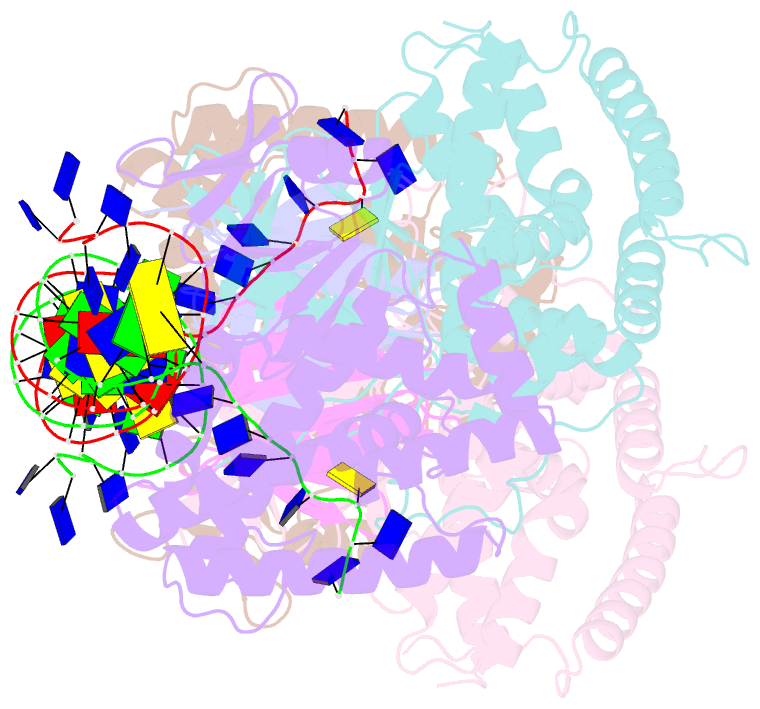
- Crystal structure of cas-DNA-pam complex
- Reference
- Wang J, Li J, Zhao H, Sheng G, Wang M, Yin M, Wang Y (2015): "Structural and Mechanistic Basis of PAM-Dependent Spacer Acquisition in CRISPR-Cas Systems." Cell, 163, 840-853. doi: 10.1016/j.cell.2015.10.008.
- Abstract
- Bacteria acquire memory of viral invaders by incorporating invasive DNA sequence elements into the host CRISPR locus, generating a new spacer within the CRISPR array. We report on the structures of Cas1-Cas2-dual-forked DNA complexes in an effort toward understanding how the protospacer is sampled prior to insertion into the CRISPR locus. Our study reveals a protospacer DNA comprising a 23-bp duplex bracketed by tyrosine residues, together with anchored flanking 3' overhang segments. The PAM-complementary sequence in the 3' overhang is recognized by the Cas1a catalytic subunits in a base-specific manner, and subsequent cleavage at positions 5 nt from the duplex boundary generates a 33-nt DNA intermediate that is incorporated into the CRISPR array via a cut-and-paste mechanism. Upon protospacer binding, Cas1-Cas2 undergoes a significant conformational change, generating a flat surface conducive to proper protospacer recognition. Here, our study provides important structure-based mechanistic insights into PAM-dependent spacer acquisition.