Summary information and primary citation
- PDB-id
- 5ds6; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (3.352 Å)
- Summary
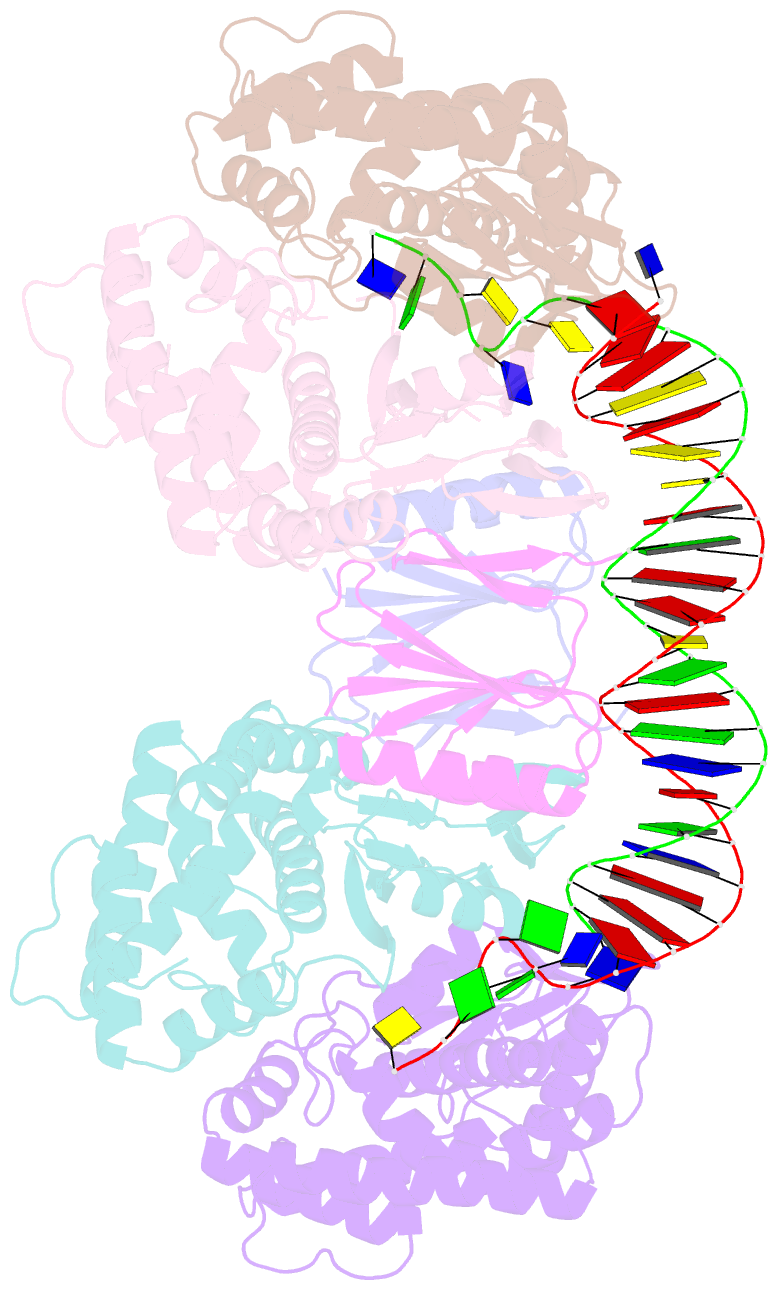
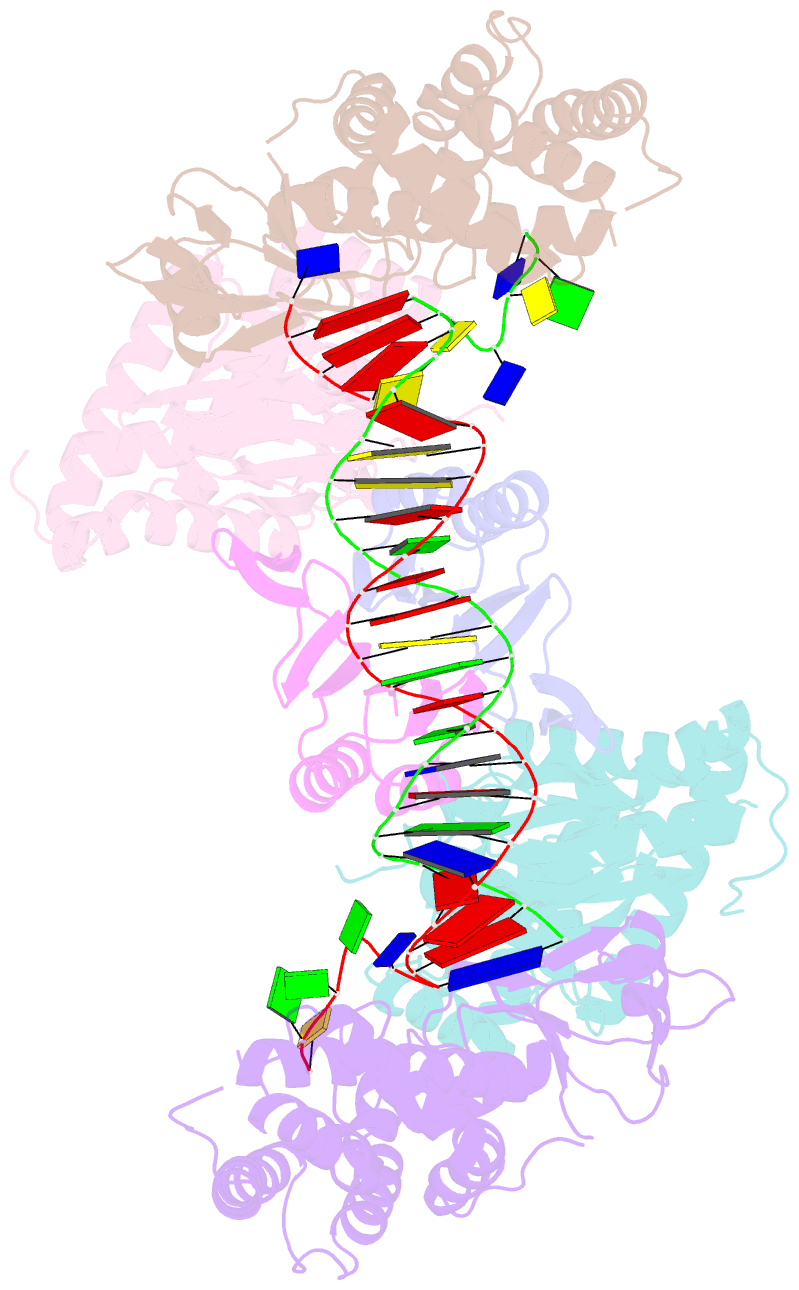
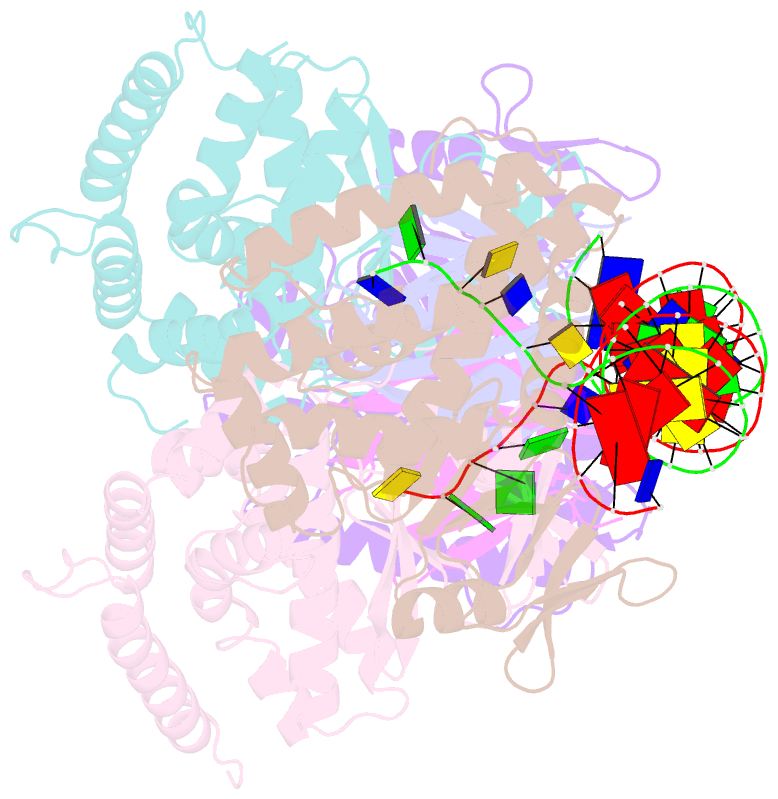
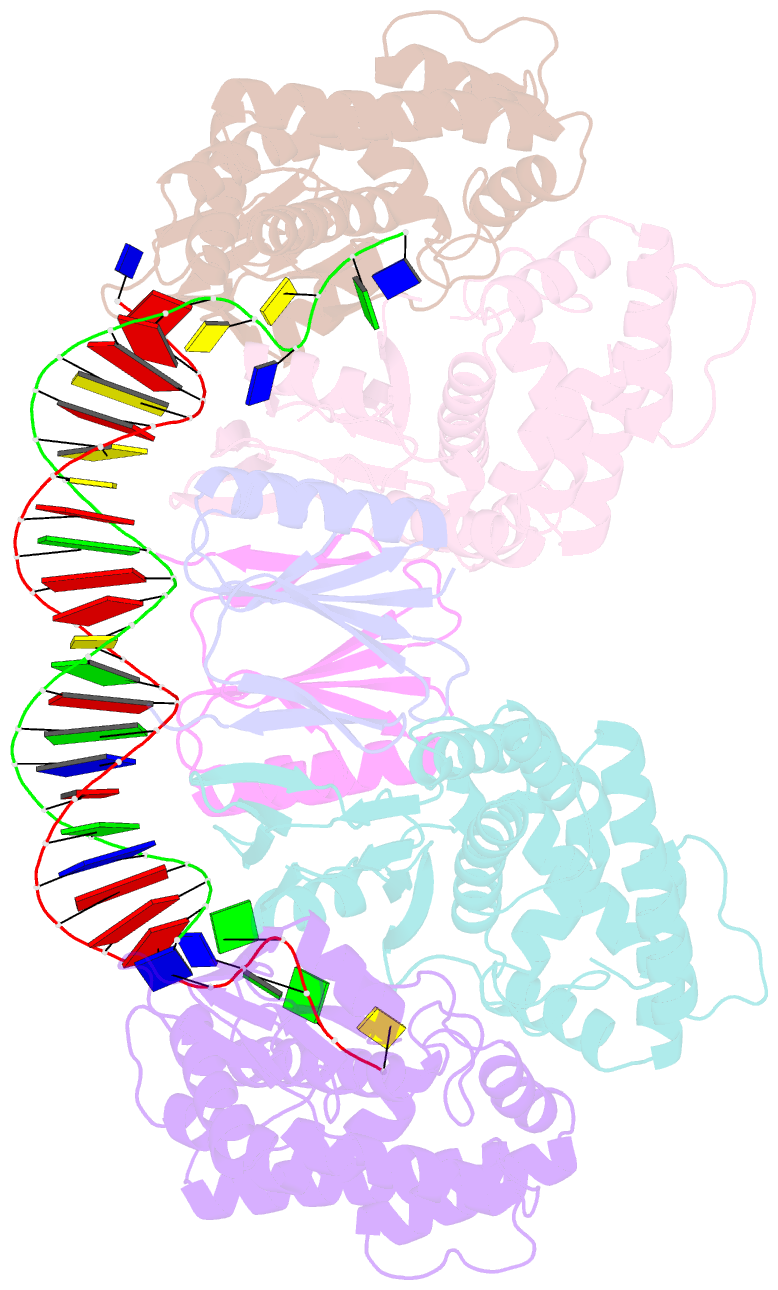
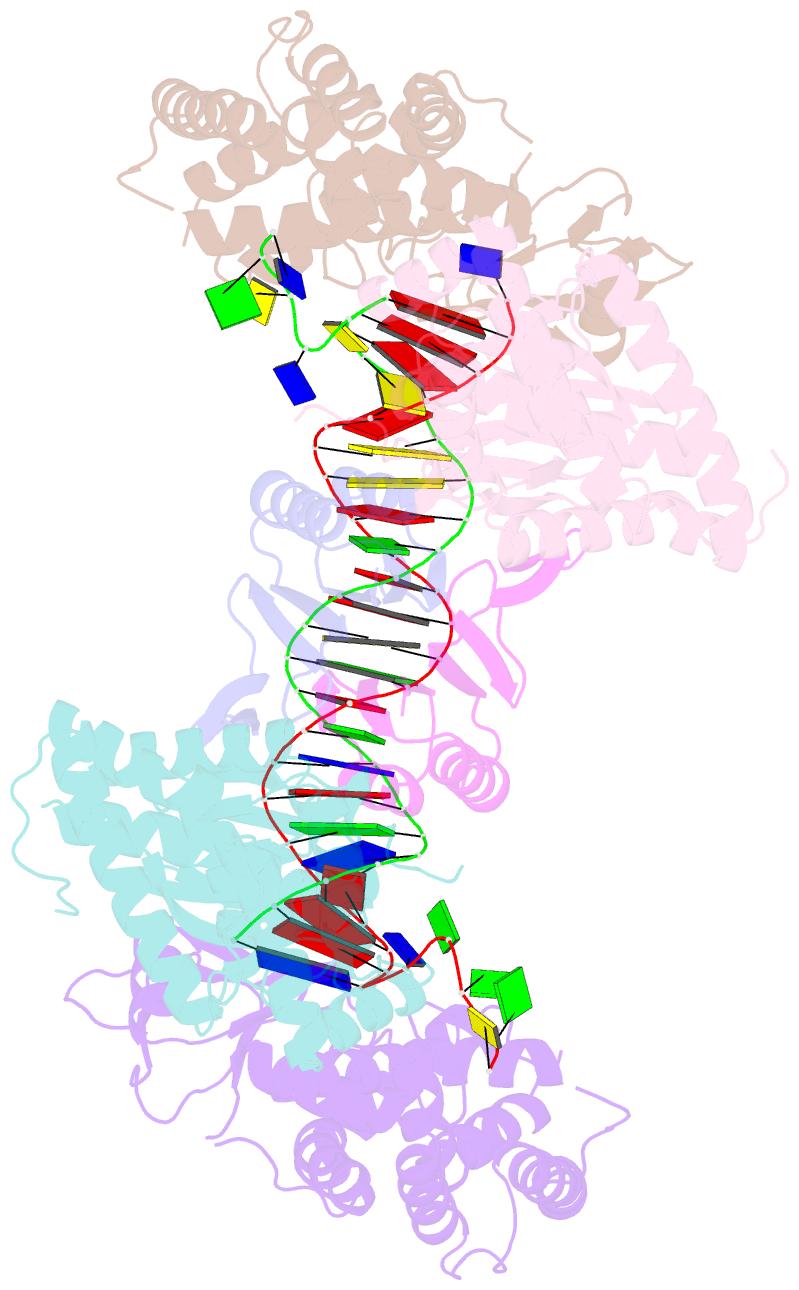
- Crystal structure the escherichia coli cas1-cas2 complex bound to protospacer DNA with splayed ends
- Reference
- Nunez JK, Harrington LB, Kranzusch PJ, Engelman AN, Doudna JA (2015): "Foreign DNA capture during CRISPR-Cas adaptive immunity." Nature, 527, 535-538. doi: 10.1038/nature15760.
- Abstract
- Bacteria and archaea generate adaptive immunity against phages and plasmids by integrating foreign DNA of specific 30-40-base-pair lengths into clustered regularly interspaced short palindromic repeat (CRISPR) loci as spacer segments. The universally conserved Cas1-Cas2 integrase complex catalyses spacer acquisition using a direct nucleophilic integration mechanism similar to retroviral integrases and transposases. How the Cas1-Cas2 complex selects foreign DNA substrates for integration remains unknown. Here we present X-ray crystal structures of the Escherichia coli Cas1-Cas2 complex bound to cognate 33-nucleotide protospacer DNA substrates. The protein complex creates a curved binding surface spanning the length of the DNA and splays the ends of the protospacer to allow each terminal nucleophilic 3'-OH to enter a channel leading into the Cas1 active sites. Phosphodiester backbone interactions between the protospacer and the proteins explain the sequence-nonspecific substrate selection observed in vivo. Our results uncover the structural basis for foreign DNA capture and the mechanism by which Cas1-Cas2 functions as a molecular ruler to dictate the sequence architecture of CRISPR loci.