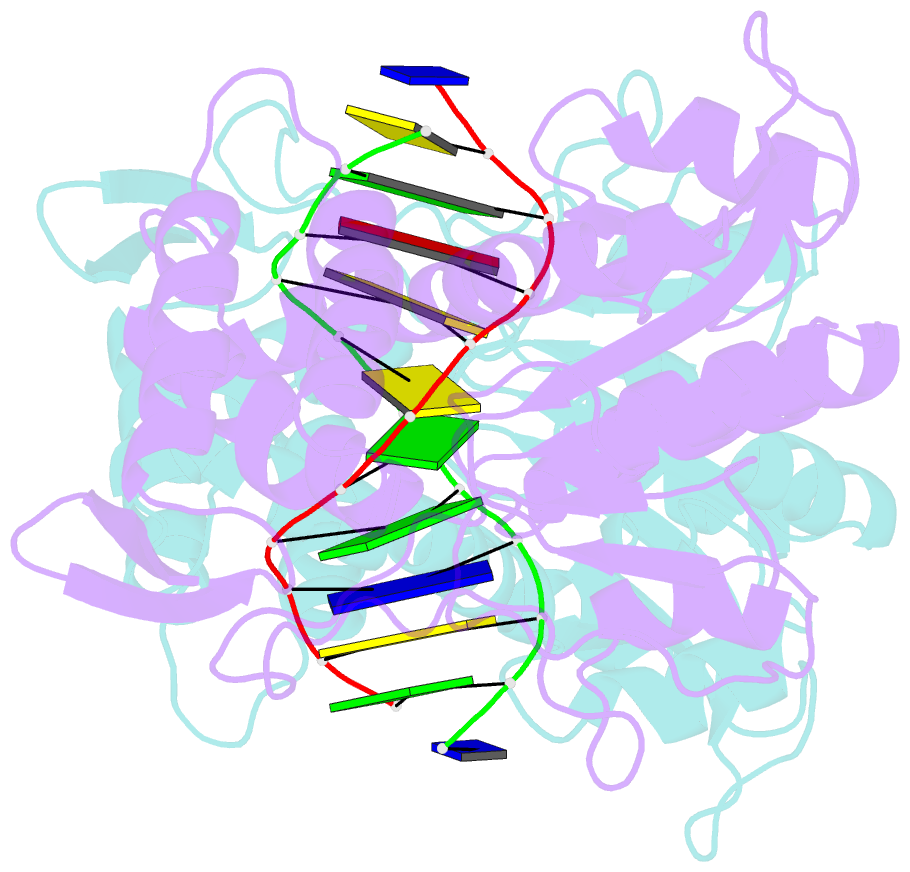
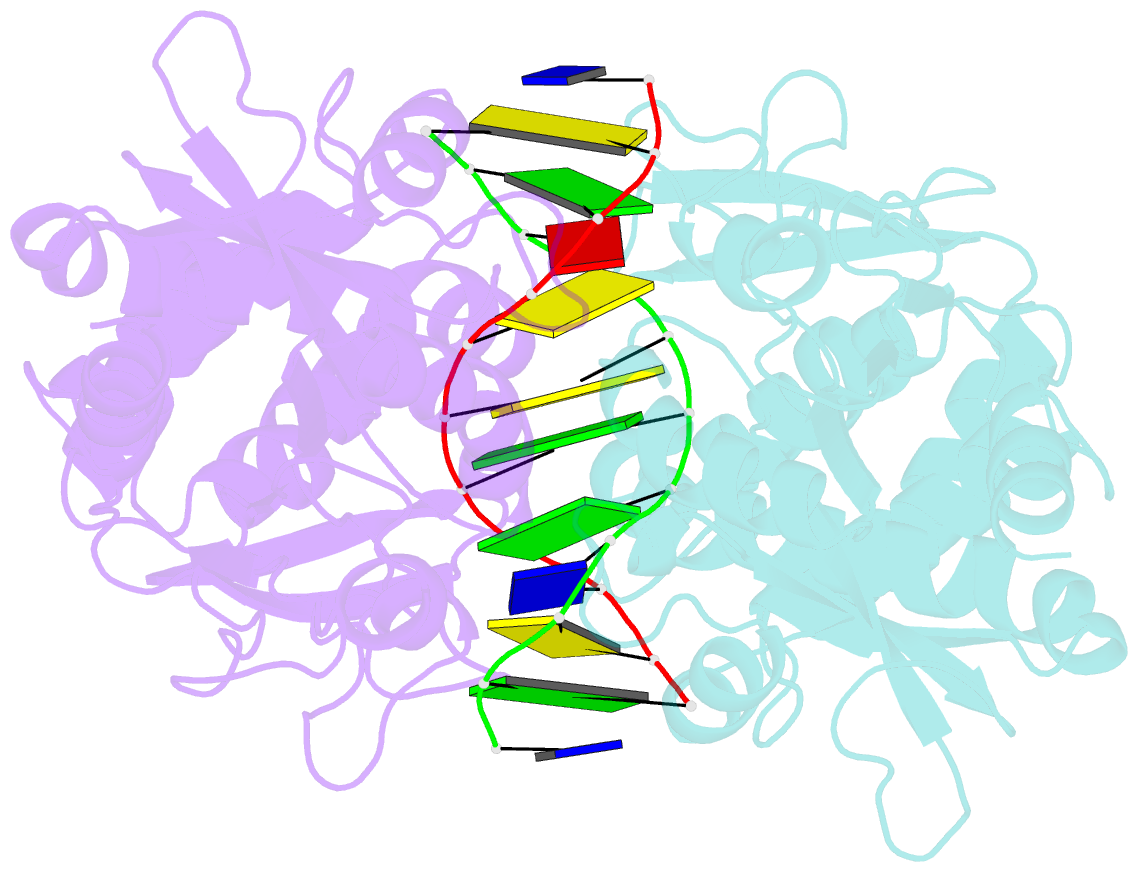
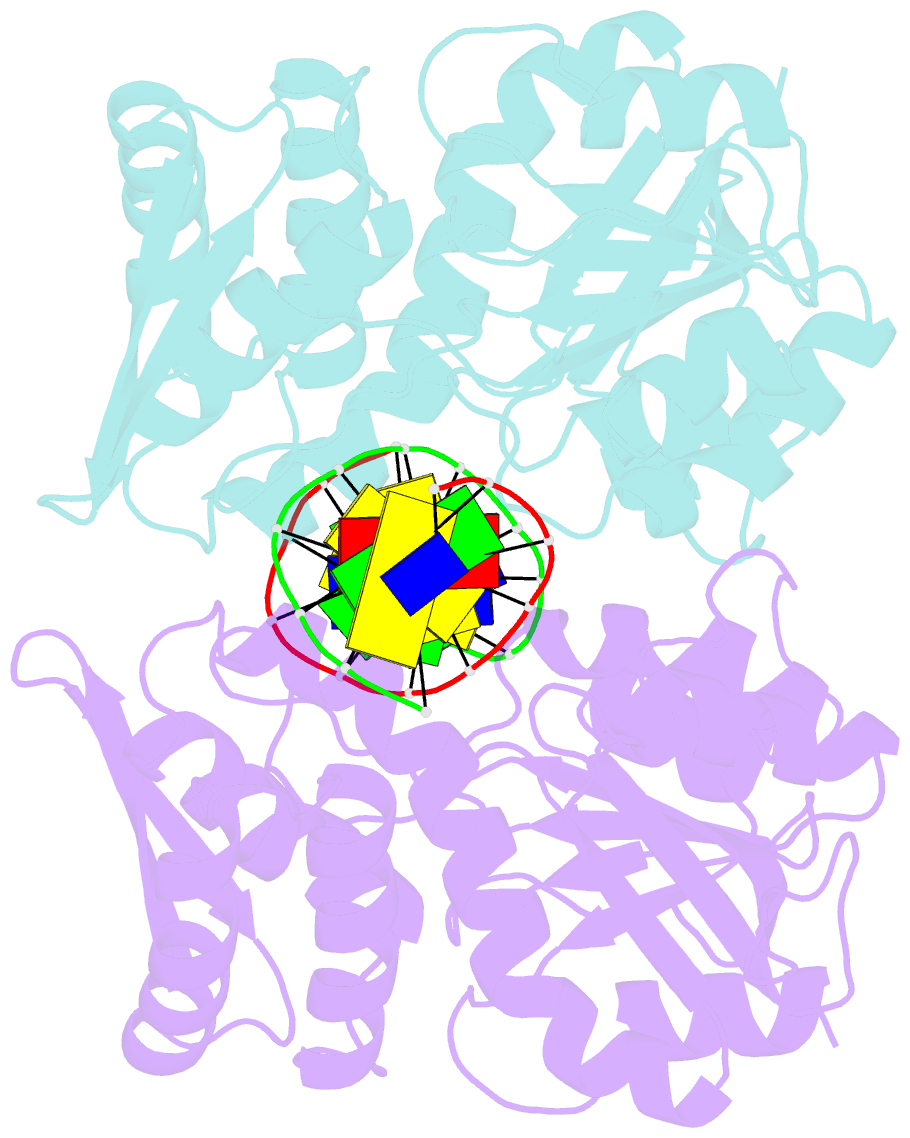
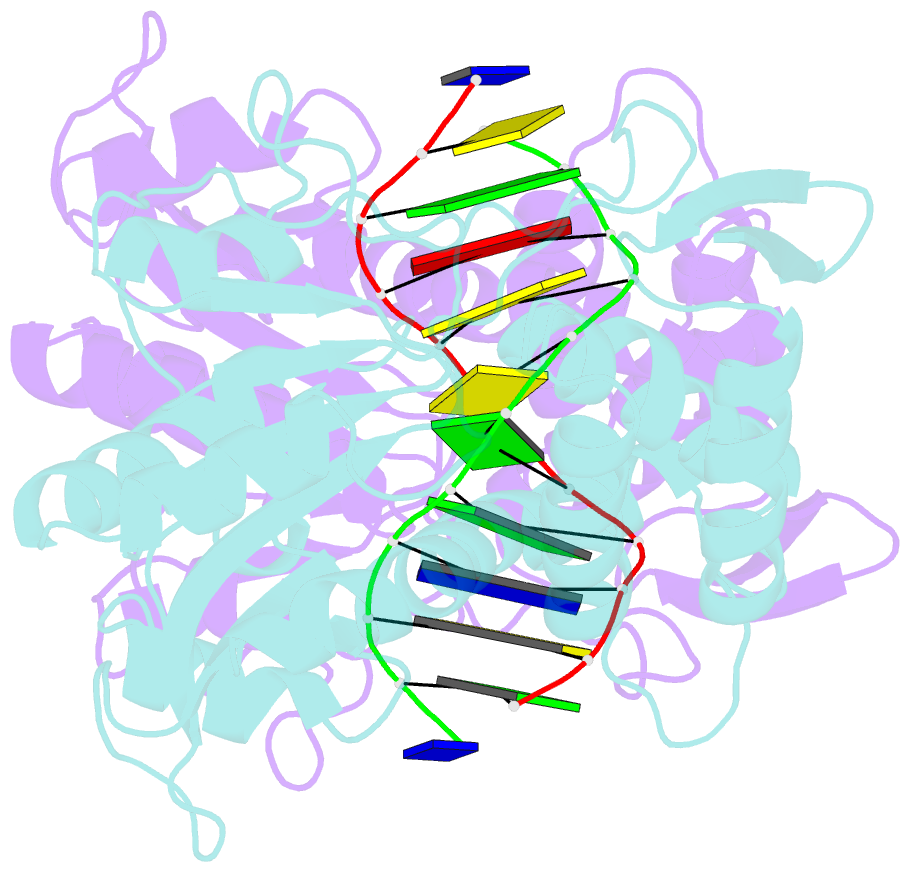
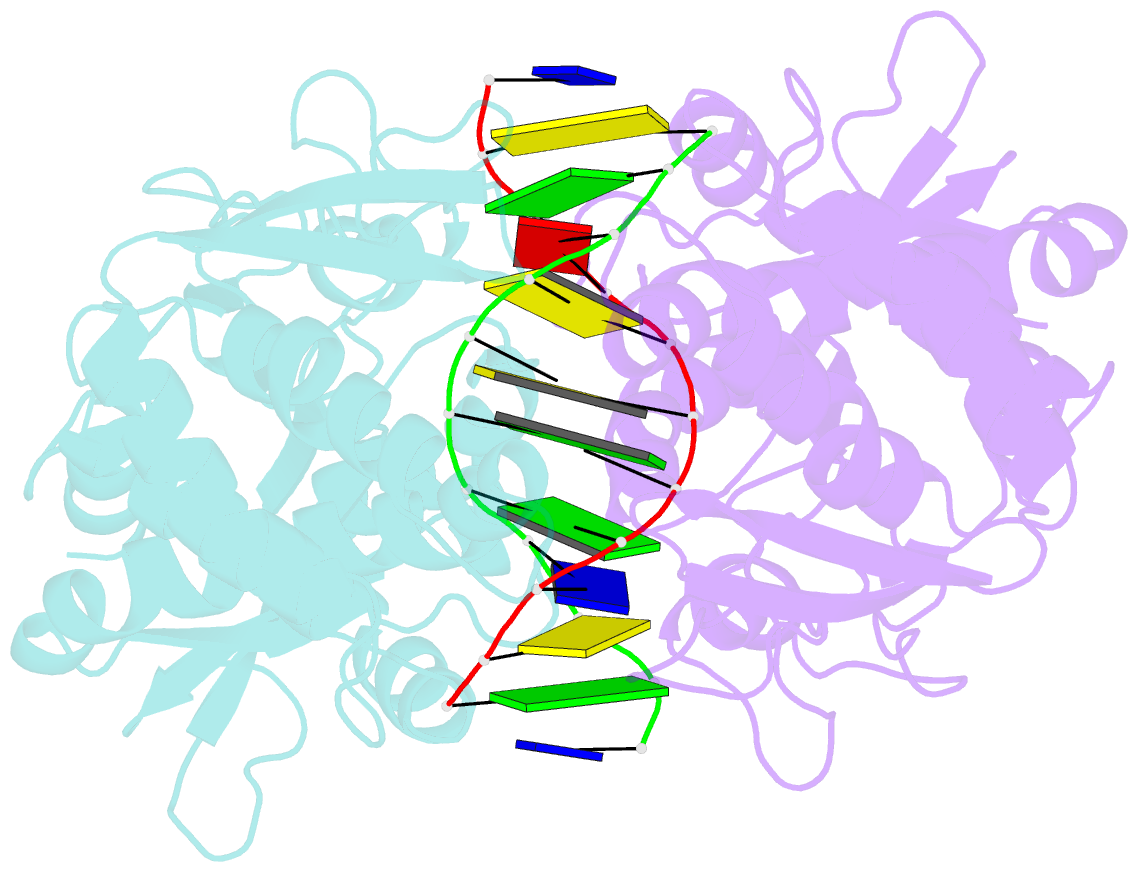
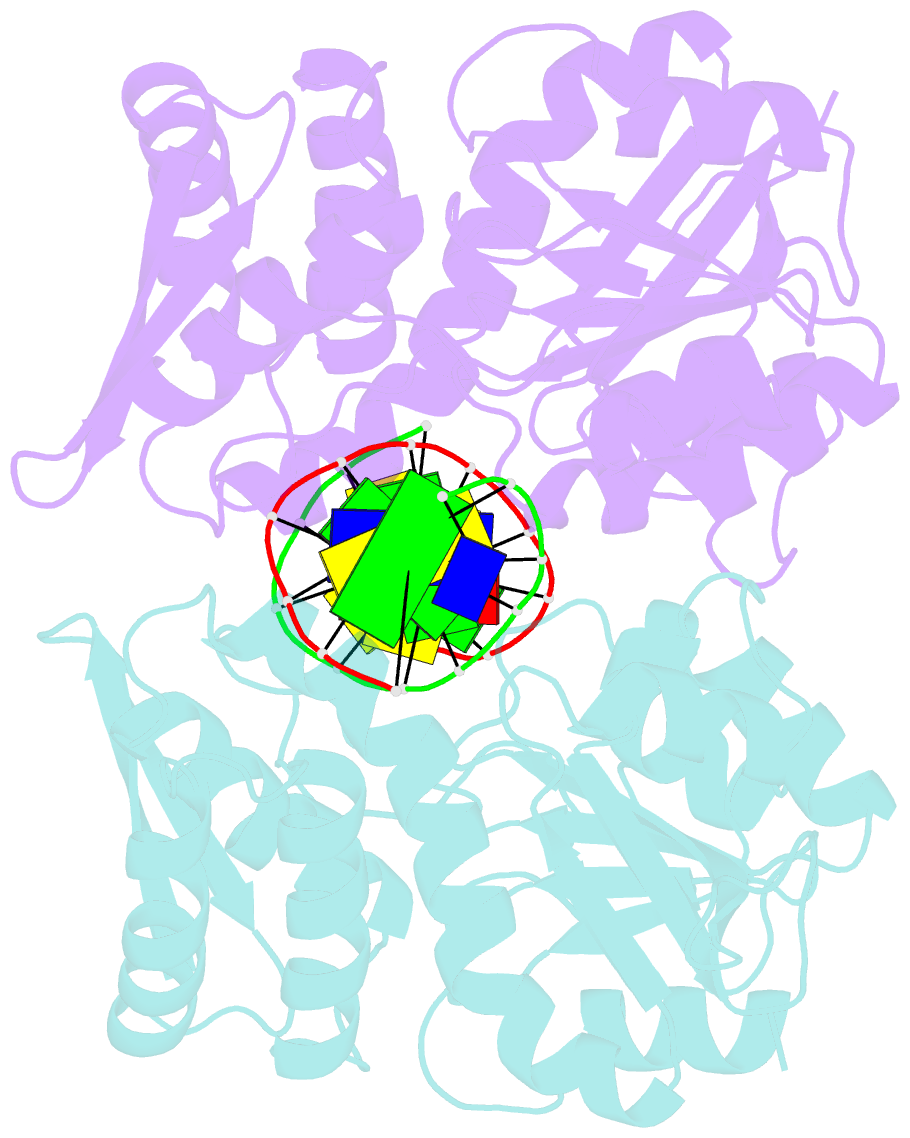
Summary information and primary citation
- PDB-id
- 5dwa; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (1.5 Å)
- Summary
- Crystal structure of pre-specific restriction endonuclease agei-DNA complex
- Reference
- Tamulaitiene G, Jovaisaite V, Tamulaitis G, Songailiene I, Manakova E, Zaremba M, Grazulis S, Xu SY, Siksnys V (2017): "Restriction endonuclease AgeI is a monomer which dimerizes to cleave DNA." Nucleic Acids Res., 45, 3547-3558. doi: 10.1093/nar/gkw1310.
- Abstract
- Although all Type II restriction endonucleases catalyze phosphodiester bond hydrolysis within or close to their DNA target sites, they form different oligomeric assemblies ranging from monomers, dimers, tetramers to higher order oligomers to generate a double strand break in DNA. Type IIP restriction endonuclease AgeI recognizes a palindromic sequence 5΄-A/CCGGT-3΄ and cuts it ('/' denotes the cleavage site) producing staggered DNA ends. Here, we present crystal structures of AgeI in apo and DNA-bound forms. The structure of AgeI is similar to the restriction enzymes that share in their target sites a conserved CCGG tetranucleotide and a cleavage pattern. Structure analysis and biochemical data indicate, that AgeI is a monomer in the apo-form both in the crystal and in solution, however, it binds and cleaves the palindromic target site as a dimer. DNA cleavage mechanism of AgeI is novel among Type IIP restriction endonucleases.