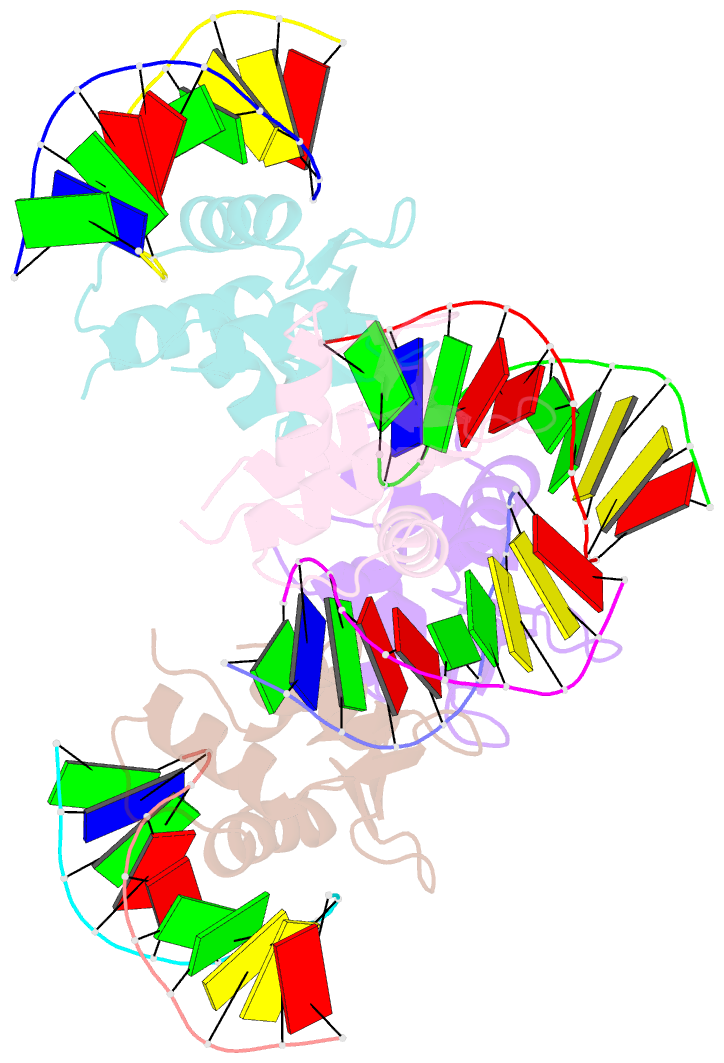
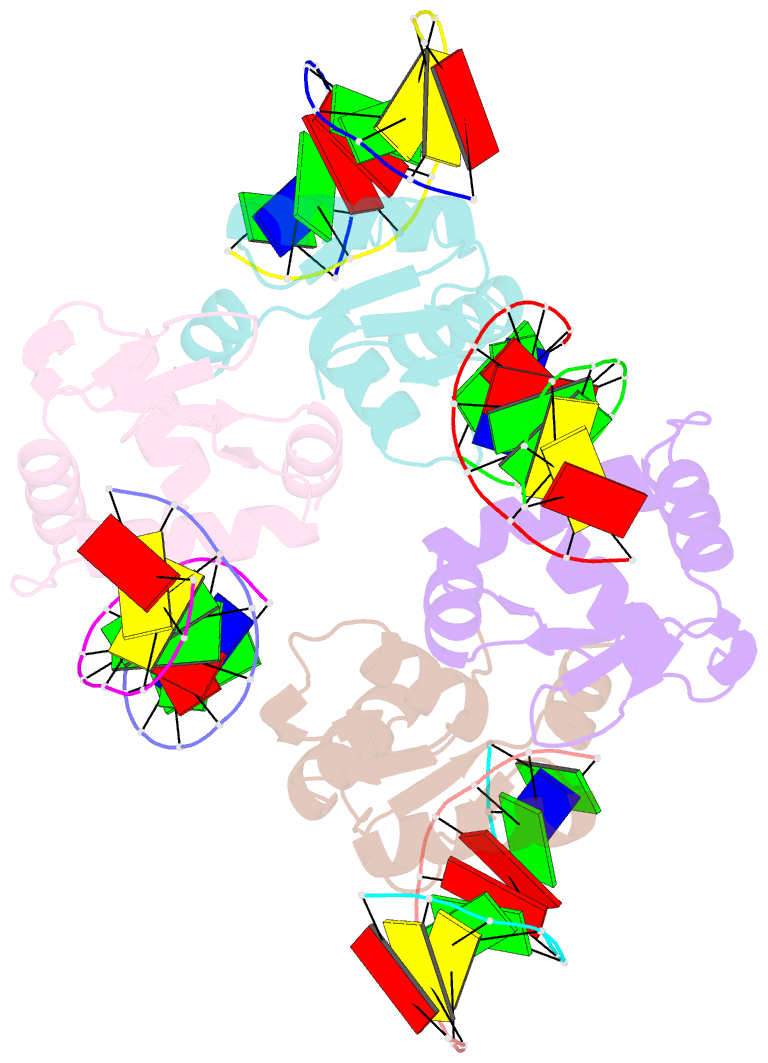
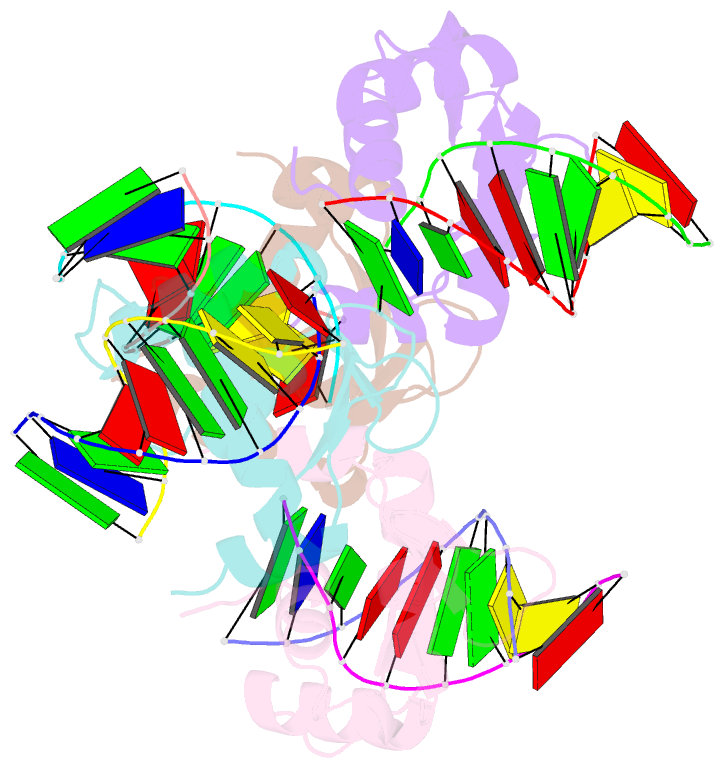
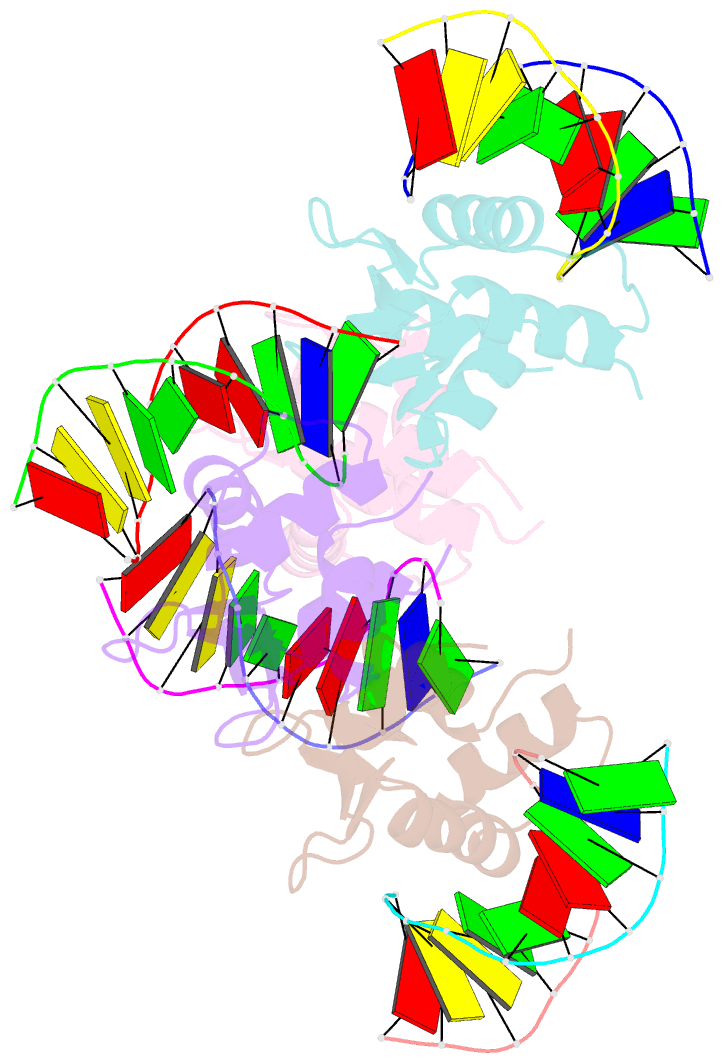
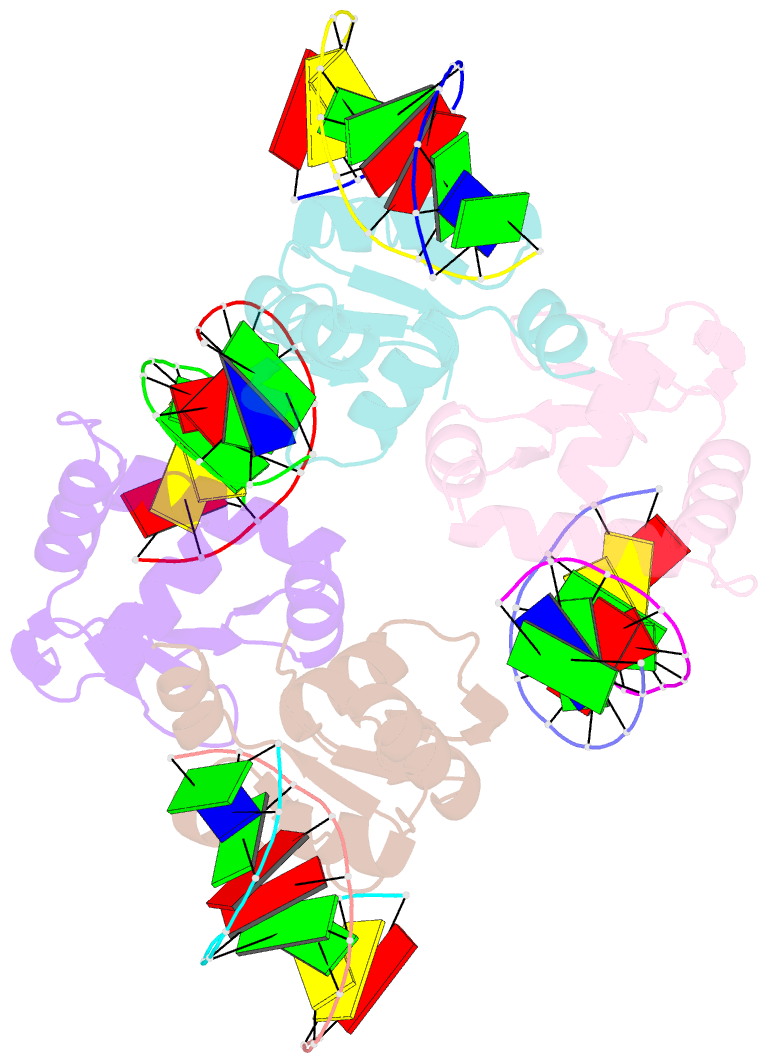
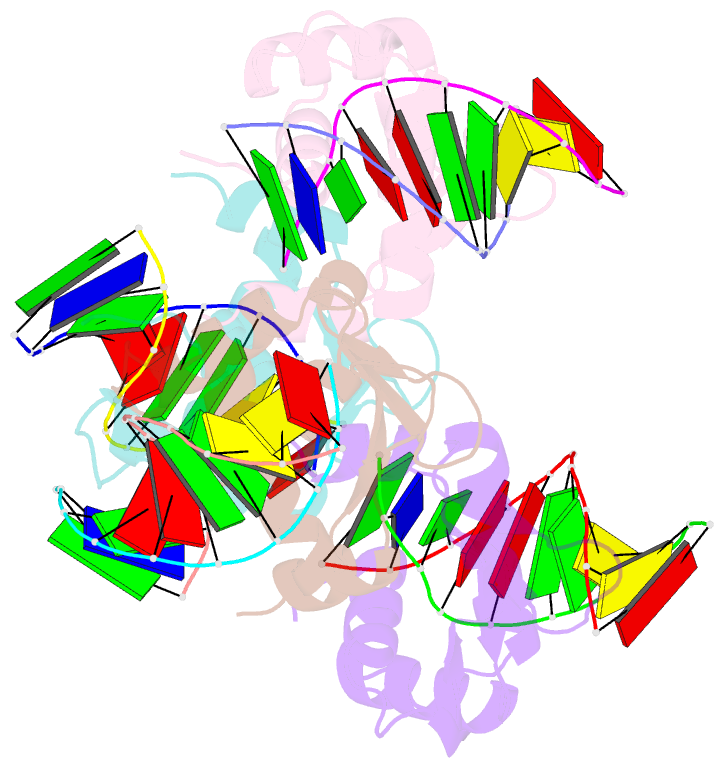
Summary information and primary citation
- PDB-id
- 5e8i; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (3.45 Å)
- Summary
- Crystal structure of the DNA binding domain of human transcription factor fli1 in complex with a 10-mer DNA accggaagtg
- Reference
- Hou C, Tsodikov OV (2015): "Structural Basis for Dimerization and DNA Binding of Transcription Factor FLI1." Biochemistry, 54, 7365-7374. doi: 10.1021/acs.biochem.5b01121.
- Abstract
- FLI1 (Friend leukemia integration 1) is a metazoan transcription factor that is upregulated in a number of cancers. In addition, rearrangements of the fli1 gene cause sarcomas, leukemias, and lymphomas. These rearrangements encode oncogenic transcription factors, in which the DNA binding domain (DBD or ETS domain) of FLI1 on the C-terminal side is fused to a part of an another protein on the N-terminal side. Such abnormal cancer cell-specific fusions retain the DNA binding properties of FLI1 and acquire non-native protein-protein or protein-nucleic acid interactions of the substituted region. As a result, these fusions trigger oncogenic transcriptional reprogramming of the host cell. Interactions of FLI1 fusions with other proteins and with itself play a critical role in the oncogenic regulatory functions, and they are currently under intense scrutiny, mechanistically and as potential novel anticancer drug targets. We report elusive crystal structures of the FLI1 DBD, alone and in complex with cognate DNA containing a GGAA recognition sequence. Both structures reveal a previously unrecognized dimer of this domain, consistent with its dimerization in solution. The homodimerization interface is helix-swapped and dominated by hydrophobic interactions, including those between two interlocking Phe362 residues. A mutation of Phe362 to an alanine disrupted the propensity of this domain to dimerize without perturbing its structure or the DNA binding function, consistent with the structural observations. We propose that FLI1 DBD dimerization plays a role in transcriptional activation and repression by FLI1 and its fusions at promoters containing multiple FLI1 binding sites.