Summary information and primary citation
- PDB-id
- 5ed4; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- transcription-DNA
- Method
- X-ray (2.4 Å)
- Summary
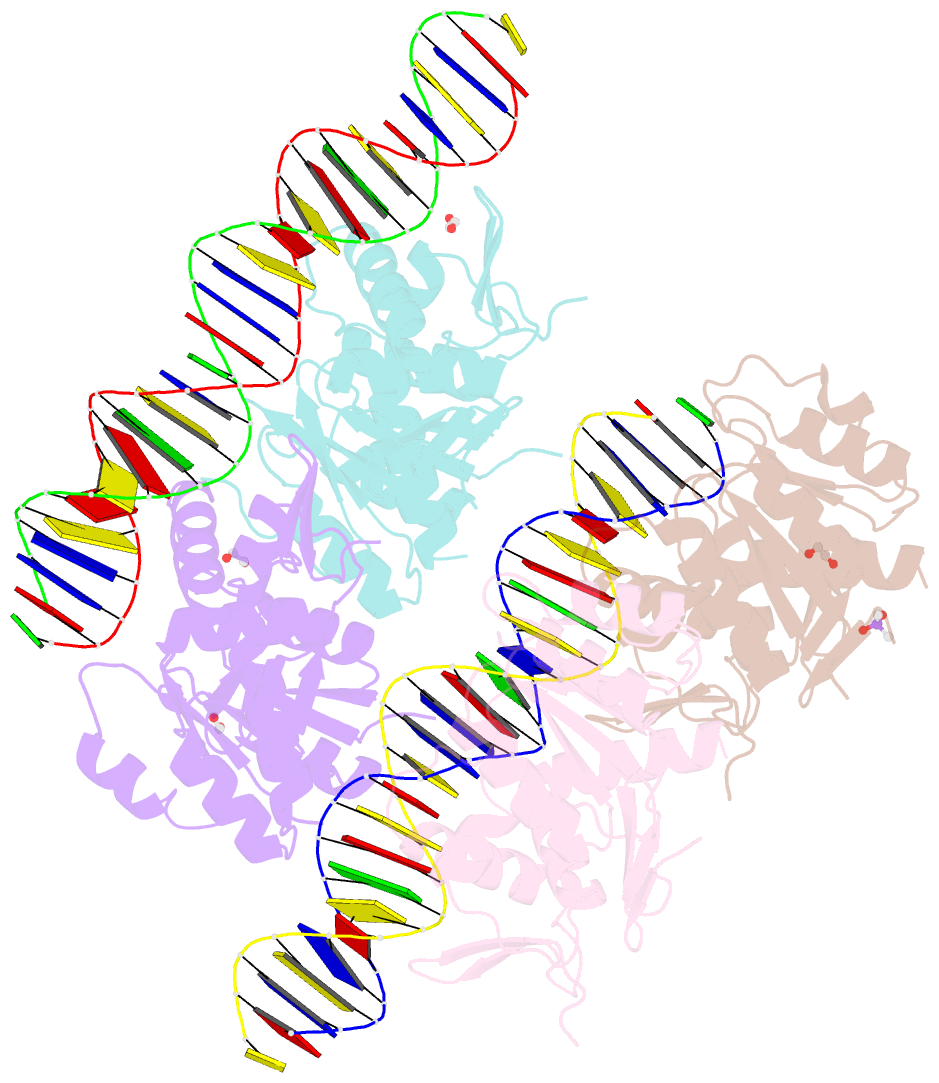
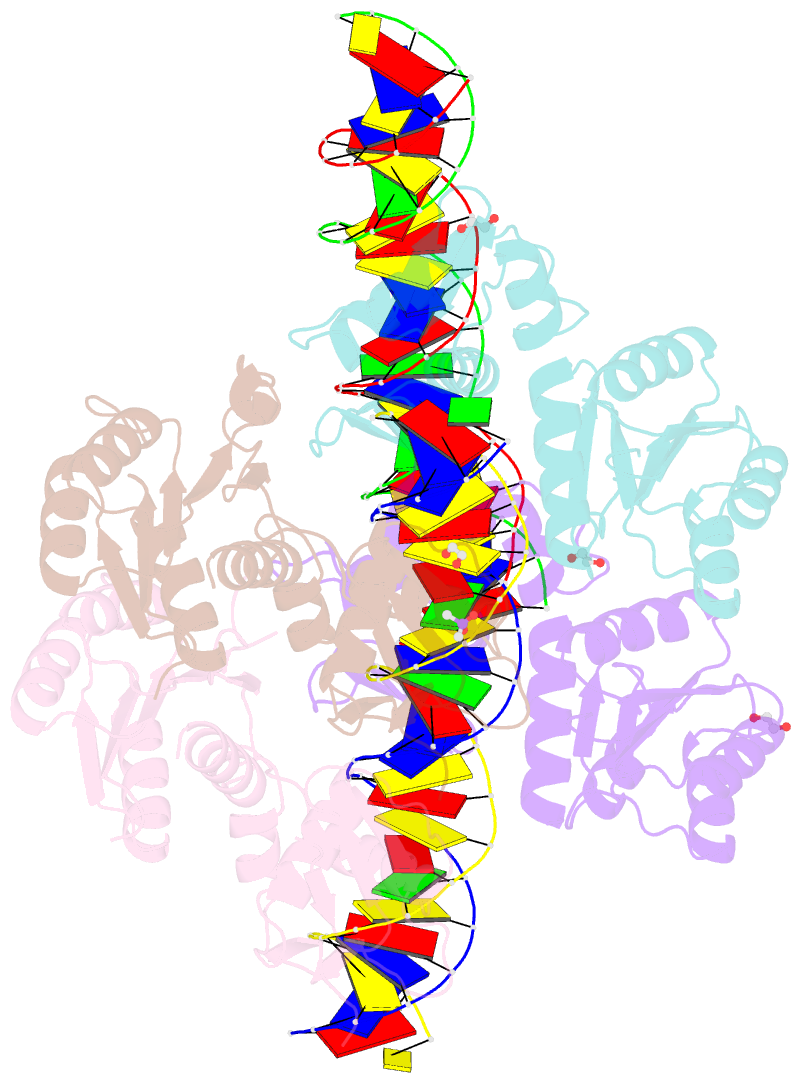
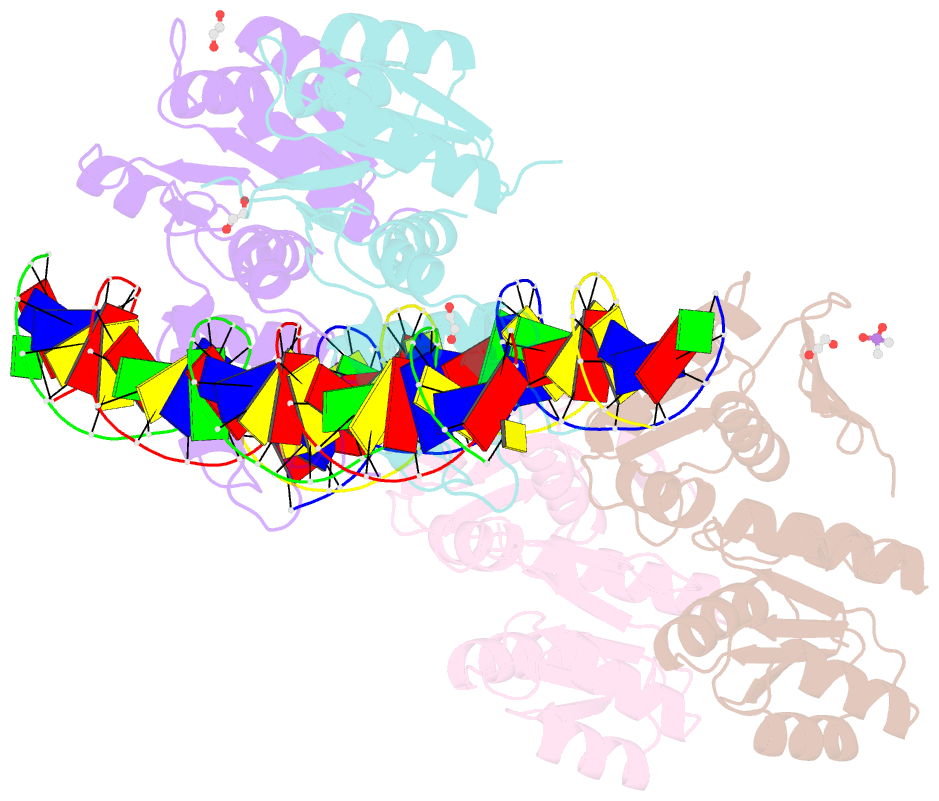
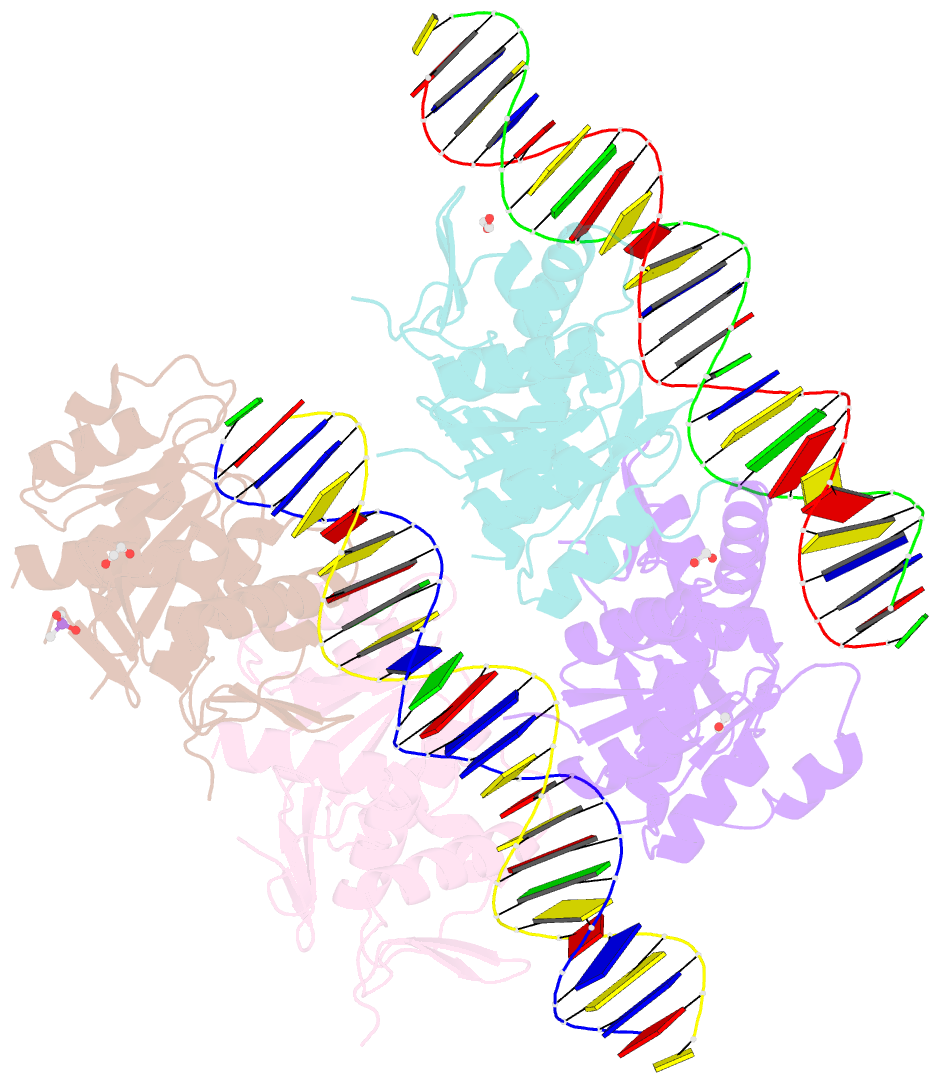
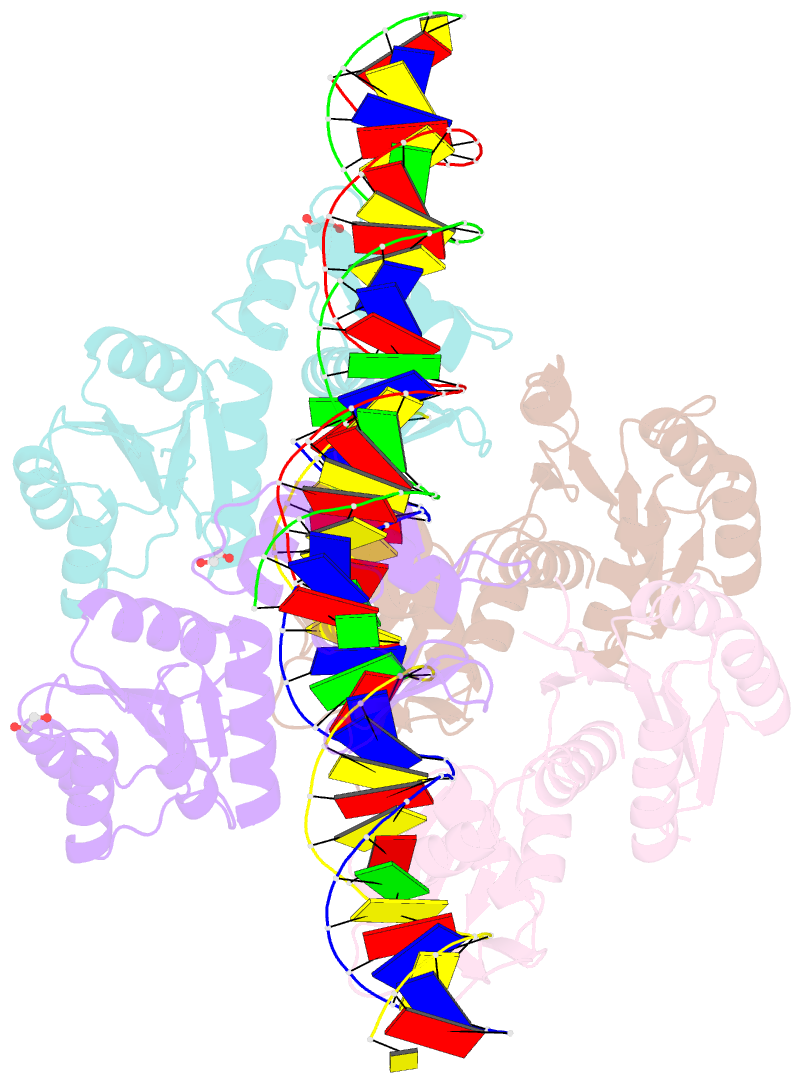
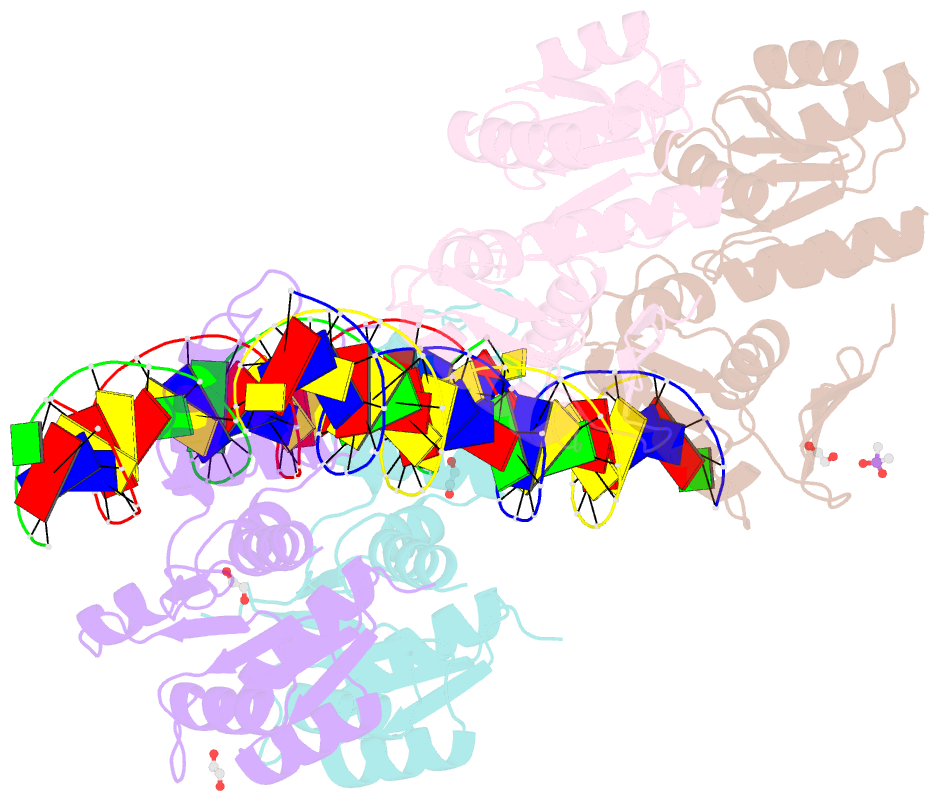
- Structure of a phop-DNA complex
- Reference
- He X, Wang L, Wang S (2016): "Structural basis of DNA sequence recognition by the response regulator PhoP in Mycobacterium tuberculosis." Sci Rep, 6, 24442. doi: 10.1038/srep24442.
- Abstract
- The transcriptional regulator PhoP is an essential virulence factor in Mycobacterium tuberculosis, and it presents a target for the development of new anti-tuberculosis drugs and attenuated tuberculosis vaccine strains. PhoP binds to DNA as a highly cooperative dimer by recognizing direct repeats of 7-bp motifs with a 4-bp spacer. To elucidate the PhoP-DNA binding mechanism, we determined the crystal structure of the PhoP-DNA complex. The structure revealed a tandem PhoP dimer that bound to the direct repeat. The surprising tandem arrangement of the receiver domains allowed the four domains of the PhoP dimer to form a compact structure, accounting for the strict requirement of a 4-bp spacer and the highly cooperative binding of the dimer. The PhoP-DNA interactions exclusively involved the effector domain. The sequence-recognition helix made contact with the bases of the 7-bp motif in the major groove, and the wing interacted with the adjacent minor groove. The structure provides a starting point for the elucidation of the mechanism by which PhoP regulates the virulence of M. tuberculosis and guides the design of screening platforms for PhoP inhibitors.