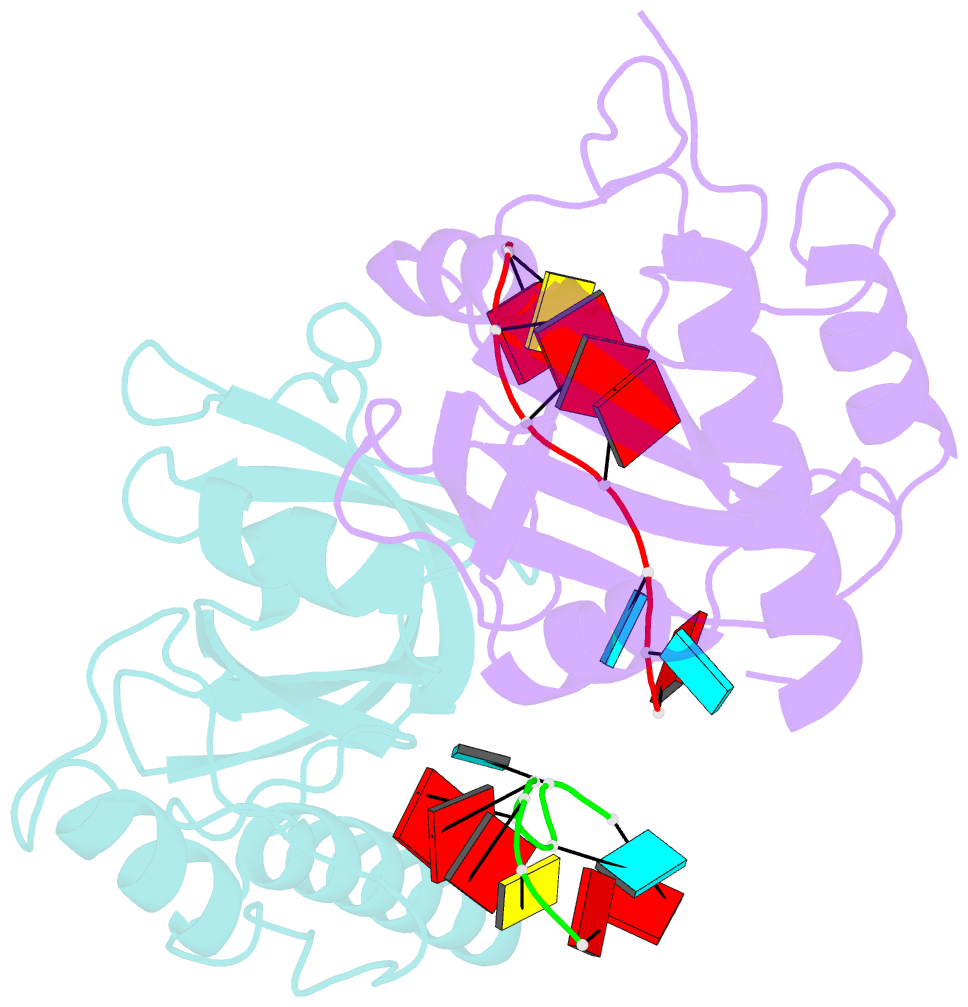
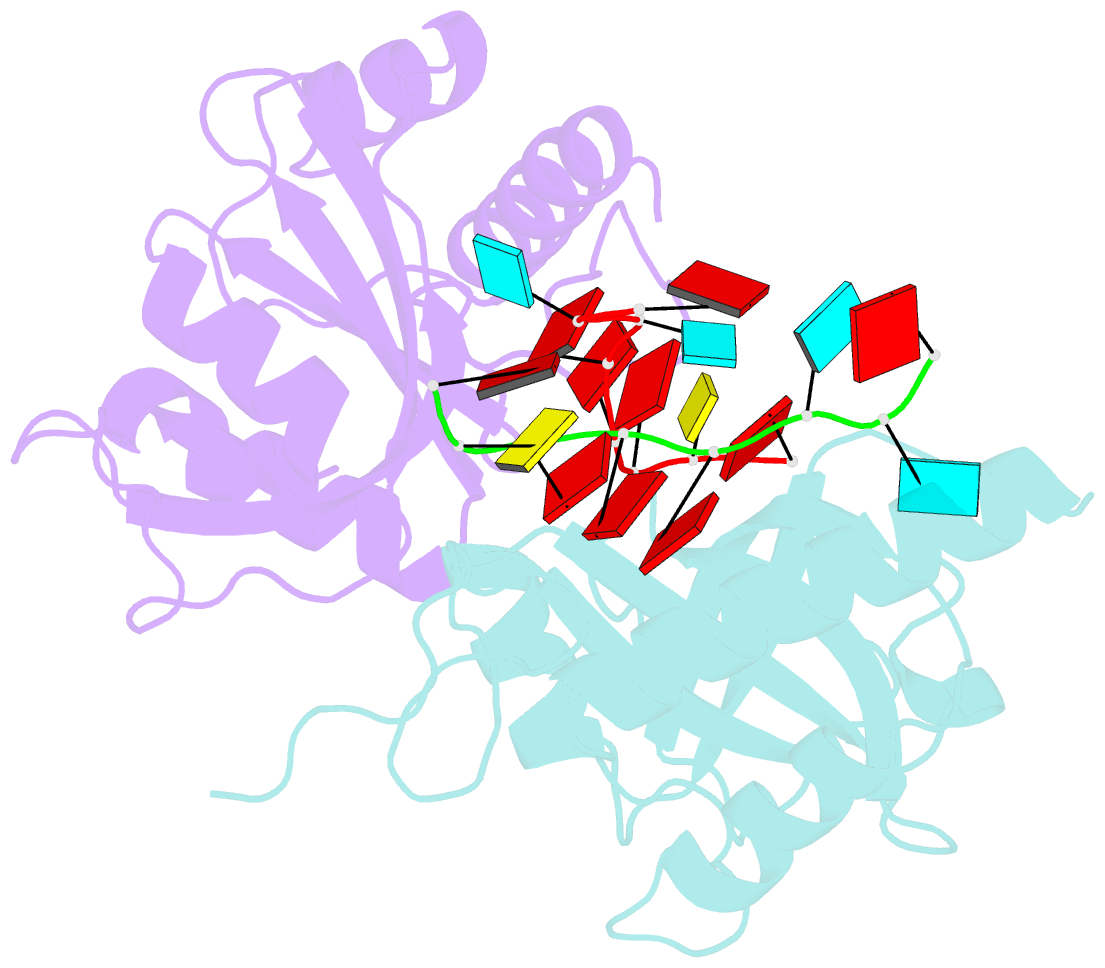
Summary information and primary citation
- PDB-id
- 5eim; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (1.54 Å)
- Summary
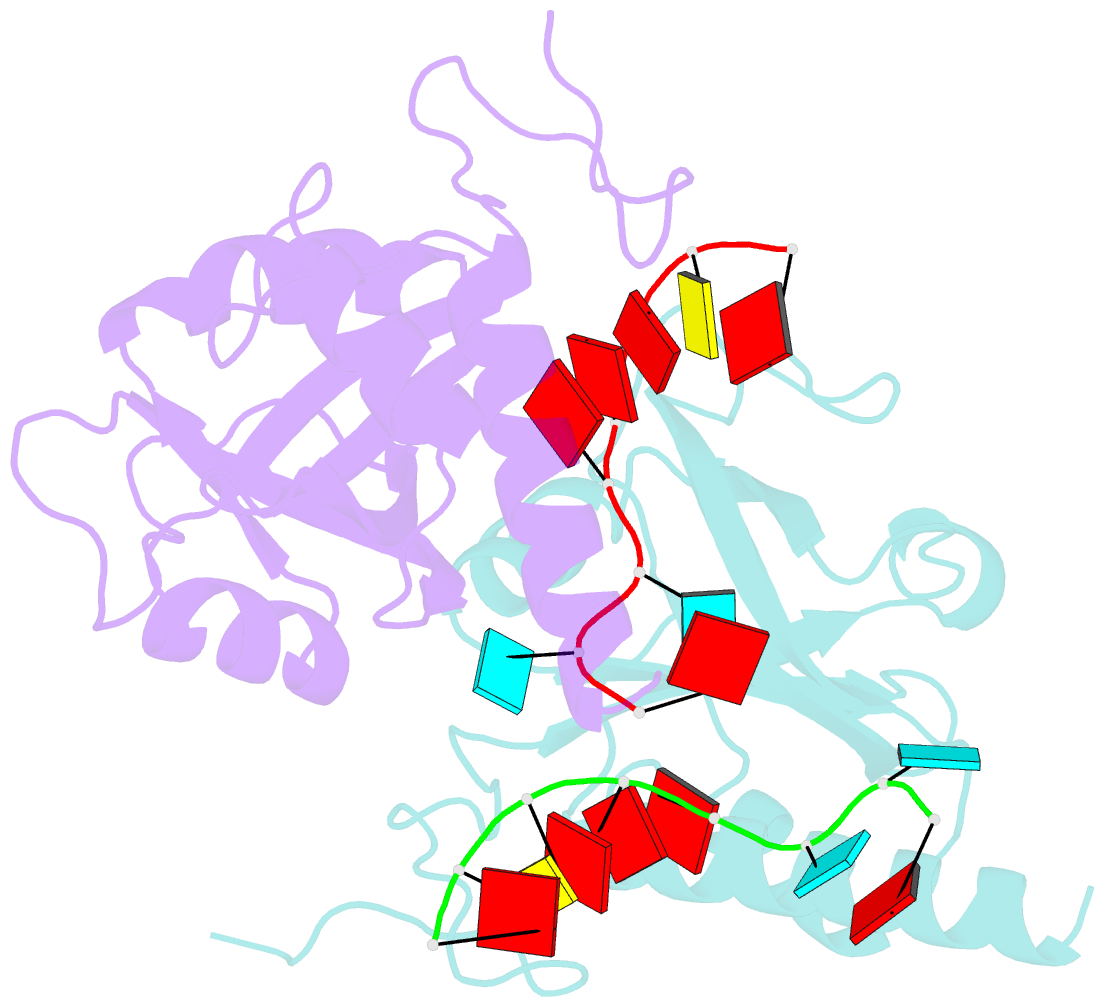
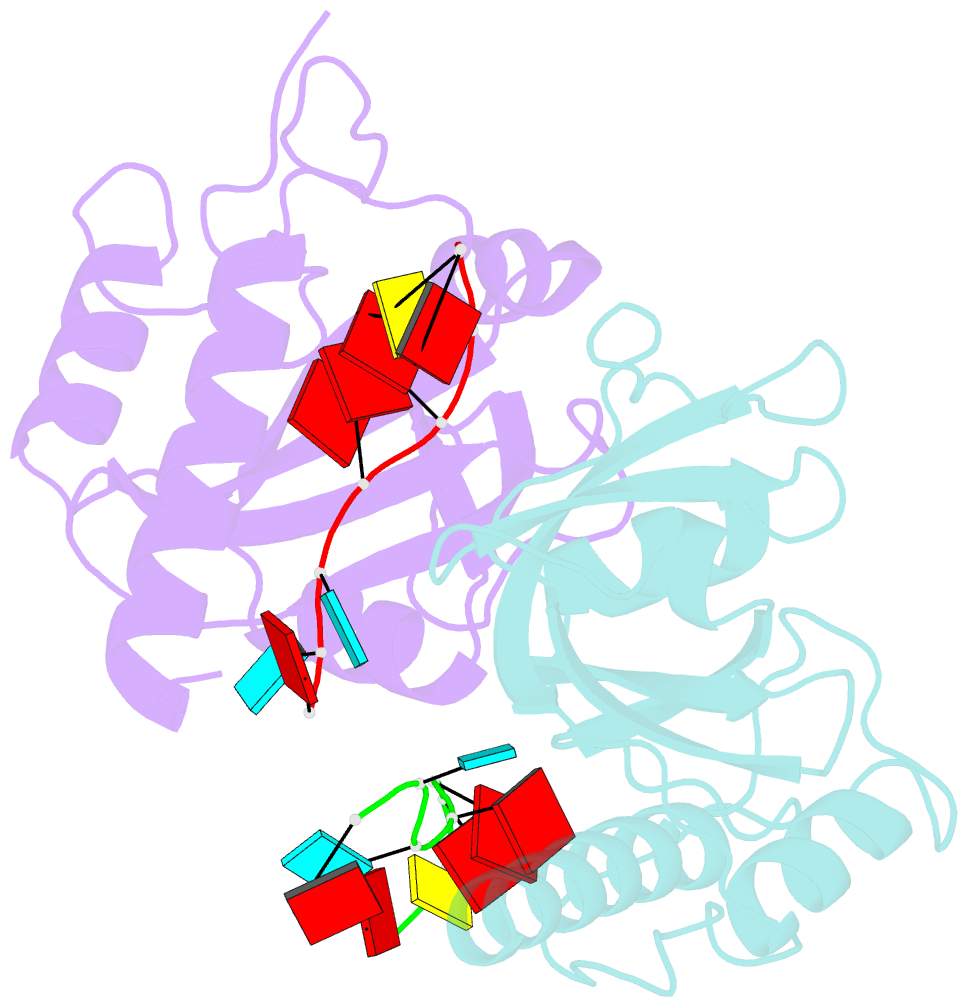
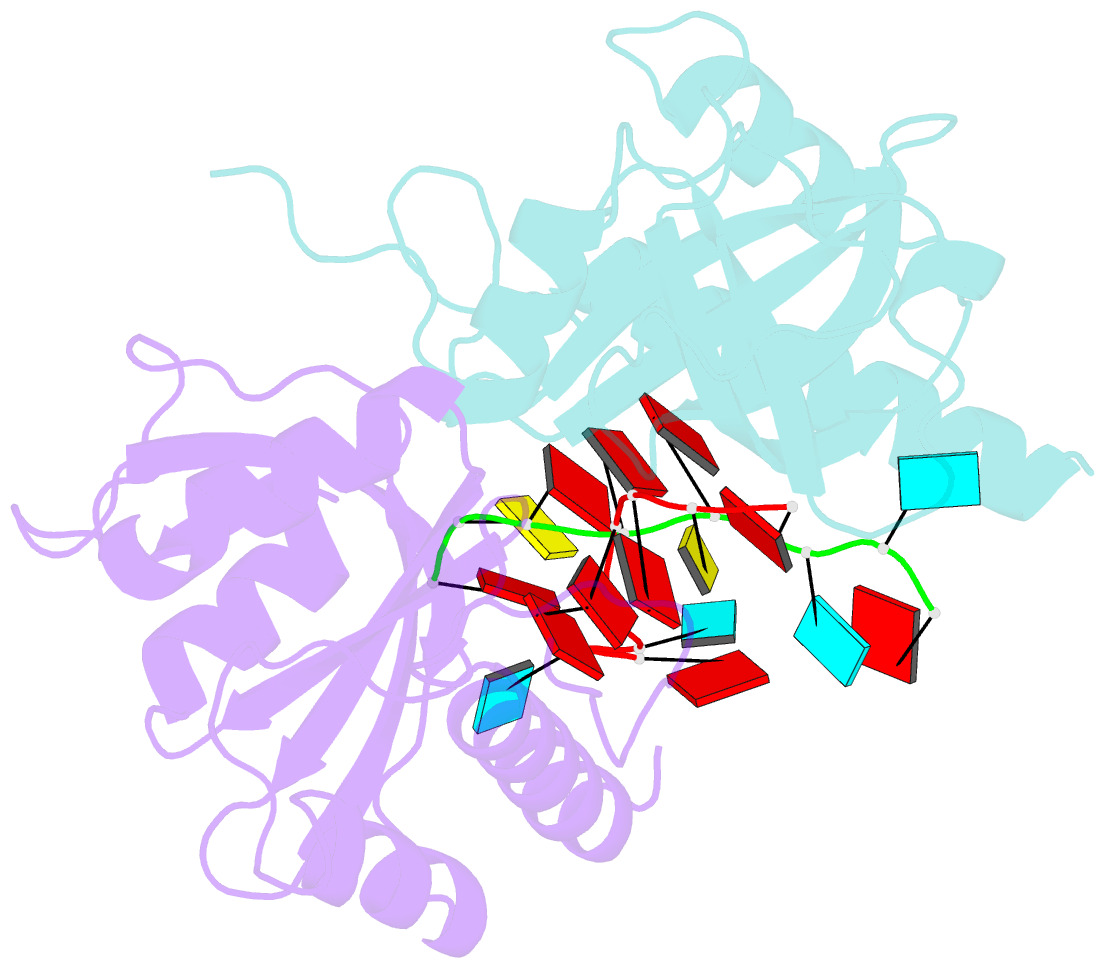
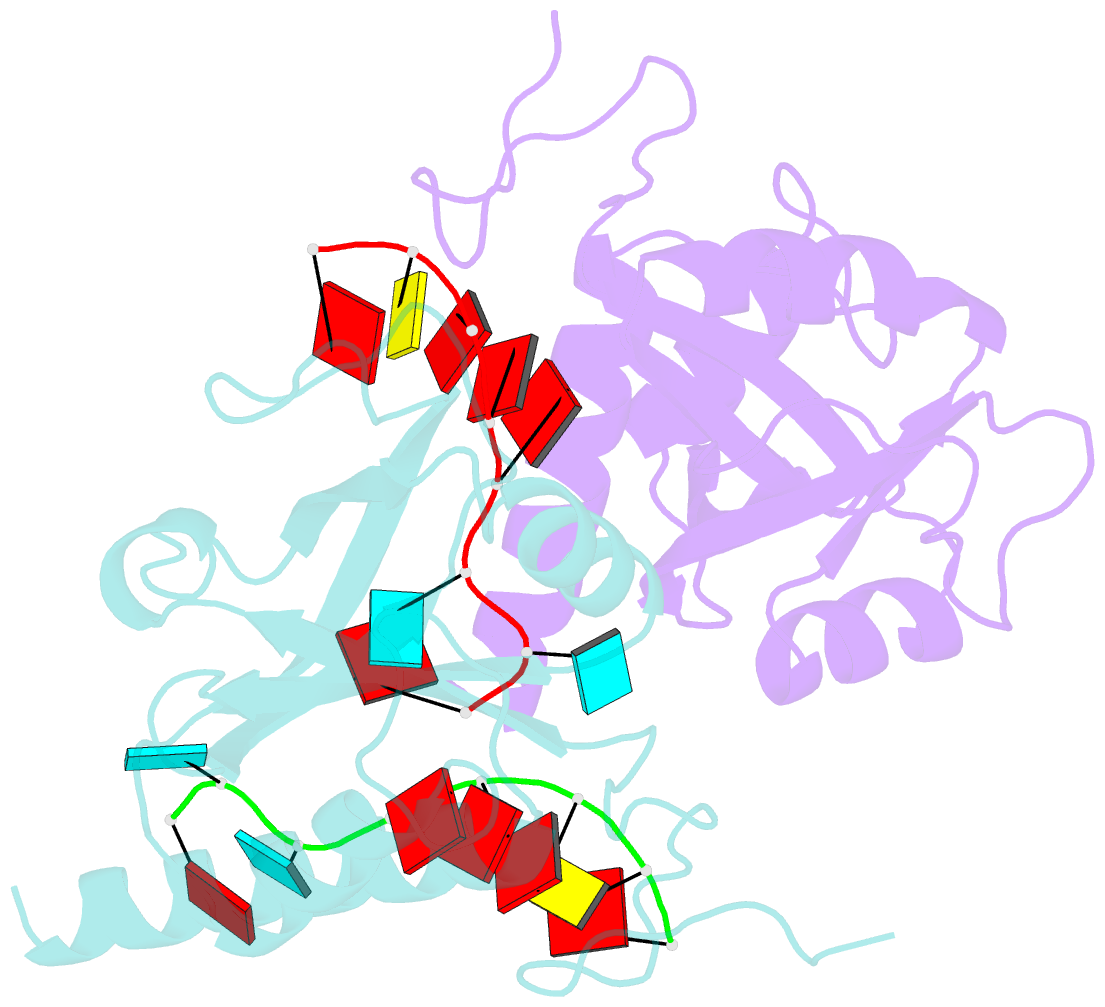
- Yth domain-containing protein mmi1 and RNA complex
- Reference
- Wu BX, Xu JH, Su SC, Liu H, Gan J, Ma JB (2017): "Structural insights into the specific recognition of DSR by the YTH domain containing protein Mmi1." Biochem. Biophys. Res. Commun., 491, 310-316. doi: 10.1016/j.bbrc.2017.07.104.
- Abstract
- Meiosis is one of the most dramatic differentiation programs accompanied by a striking change in gene expression profiles in fission yeast Schizosaccharomyces pombe. Whereas a number of meiosis-specific transcripts are expressed untimely in mitotic cells, and the entry of meiosis will be blocked as the accumulation of meiosis-specific mRNAs in the mitotic cells. A YTH domain containing protein Mmi1 was identified as a pivotal effector in a post-transcriptional event termed selective elimination of meiosis-specific mRNAs. Mmi1 can recognize and bind a class of meiosis-specific transcripts expressed inappropriately in mitotic cells, which all contain a conservative region called DSR, as a mark to remove them in cooperation with nuclear exosomes. Here we report the 1.6 Å resolution crystal structure of the Mmi1-YTH domain in complex with a high consensus hexanucleotide motif, which is multiple copied in the DSR region. Our structure observations, supported by site-directed mutations of key residues illustrate the mechanism for specific recognition of DSR-RNA by Mmi1. Moreover, different from other YTH domain family proteins, Mmi1-YTH domain has a distinctive RNA-binding properties although it has a similar fold as other ones.