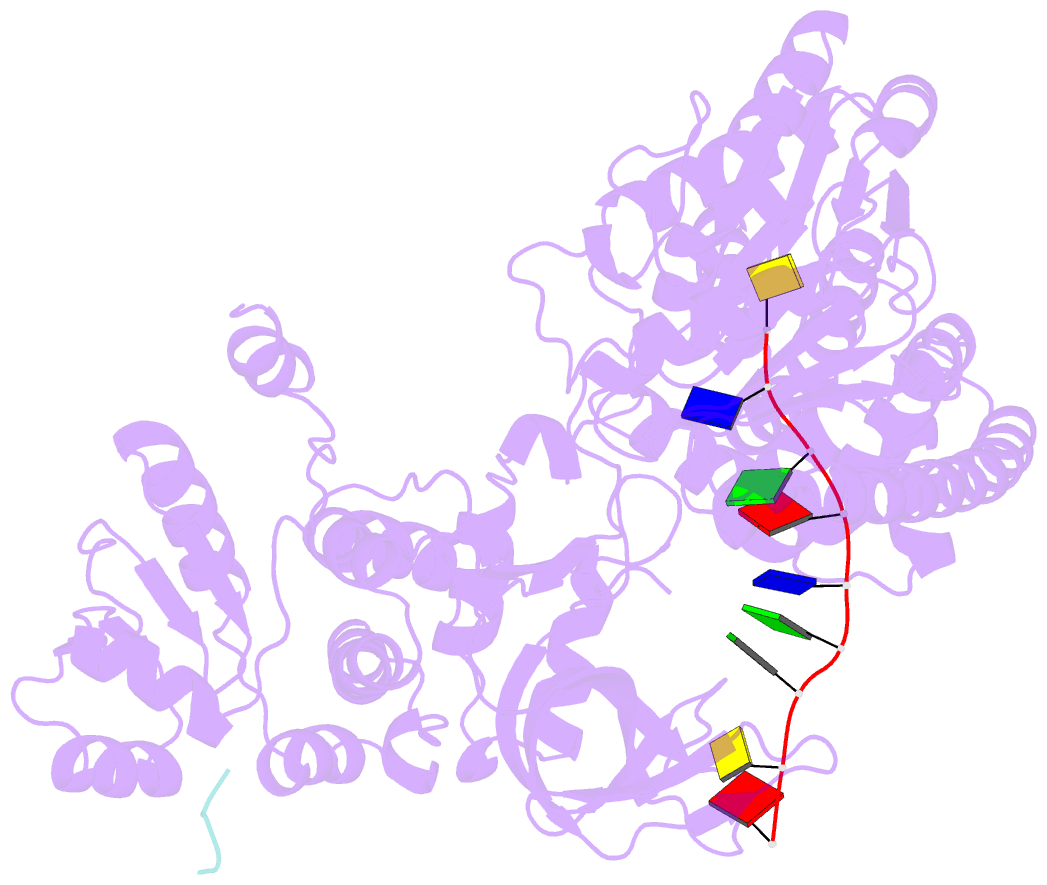
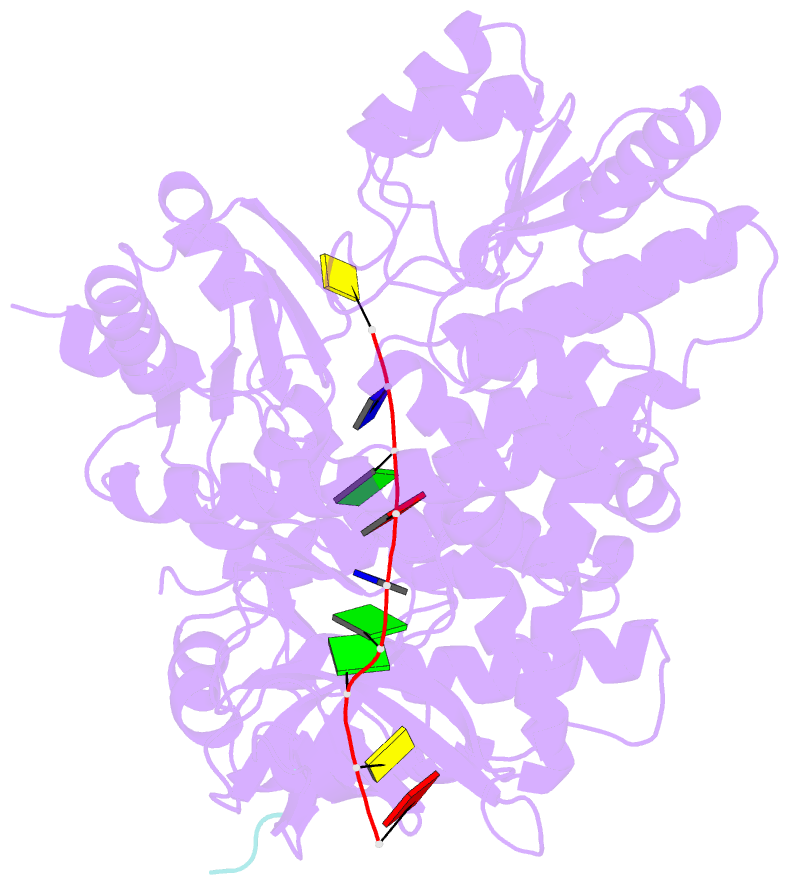
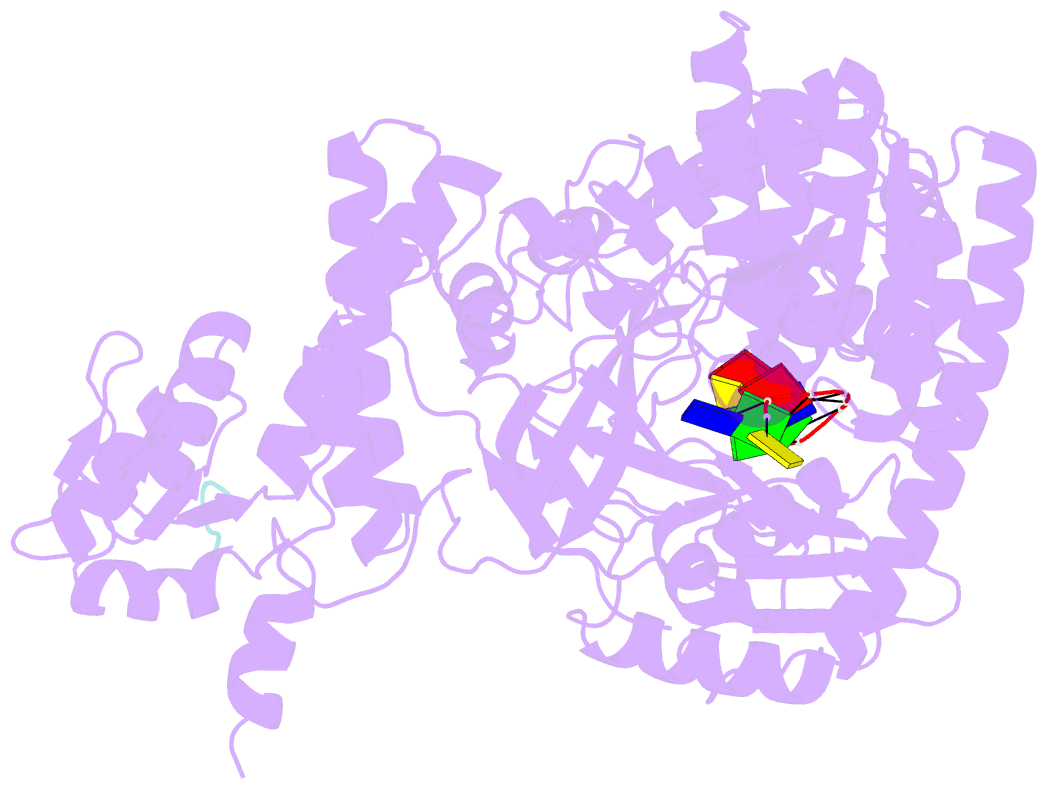
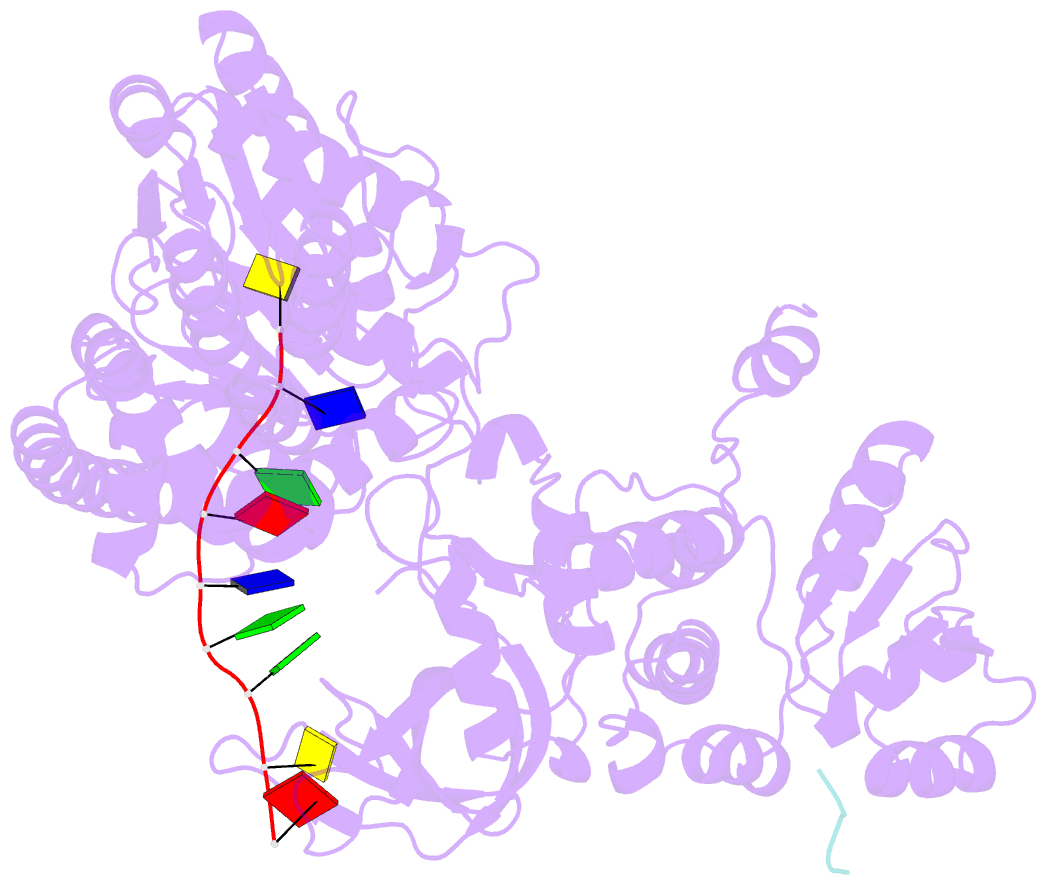
Summary information and primary citation
- PDB-id
- 5f56; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- DNA binding protein-DNA
- Method
- X-ray (2.3 Å)
- Summary
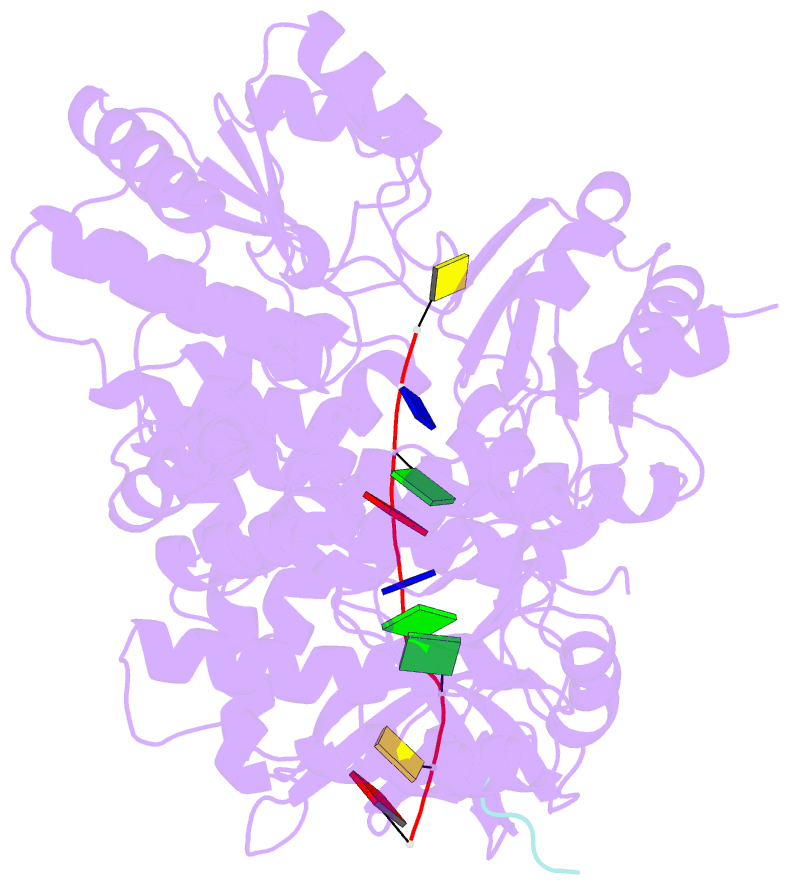
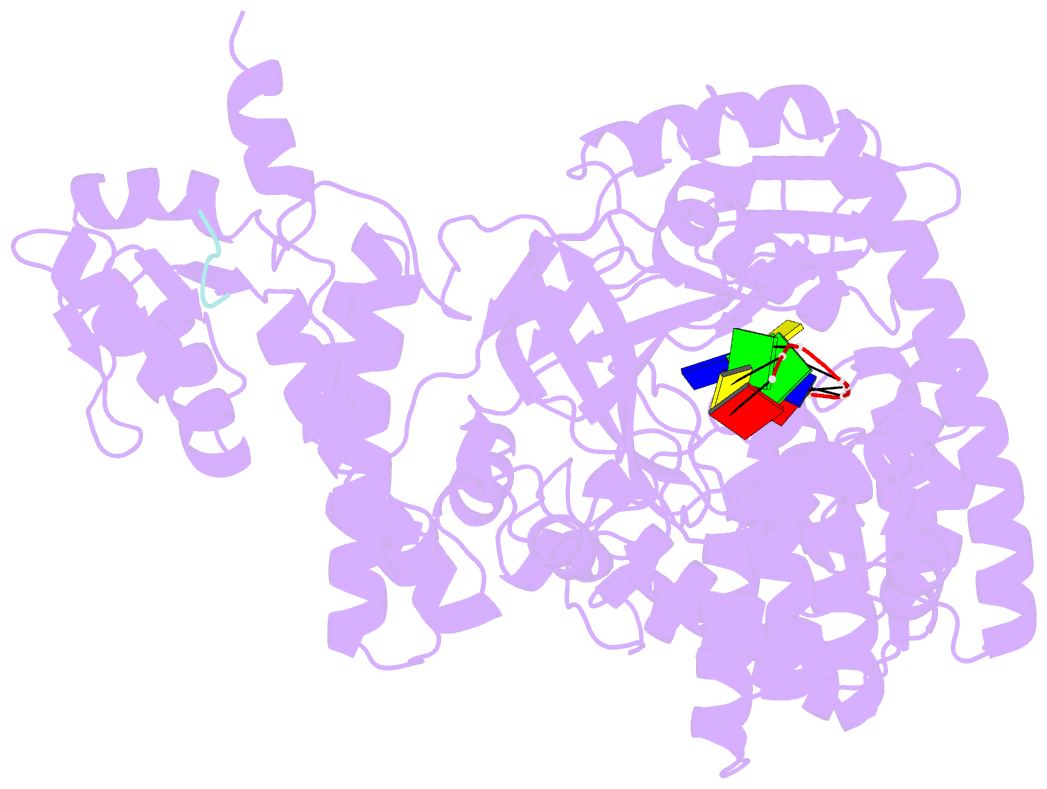
- Structure of recj complexed with DNA and ssb-ct
- Reference
- Cheng K, Xu H, Chen X, Wang L, Tian B, Zhao Y, Hua Y (2016): "Structural basis for DNA 5 -end resection by RecJ." Elife, 5, e14294. doi: 10.7554/eLife.14294.
- Abstract
- The resection of DNA strand with a 5´ end at double-strand breaks is an essential step in recombinational DNA repair. RecJ, a member of DHH family proteins, is the only 5´ nuclease involved in the RecF recombination pathway. Here, we report the crystal structures of Deinococcus radiodurans RecJ in complex with deoxythymidine monophosphate (dTMP), ssDNA, the C-terminal region of single-stranded DNA-binding protein (SSB-Ct) and a mechanistic insight into the RecF pathway. A terminal 5´-phosphate-binding pocket above the active site determines the 5´-3´ polarity of the deoxy-exonuclease of RecJ; a helical gateway at the entrance to the active site admits ssDNA only; and the continuous stacking interactions between protein and nine nucleotides ensure the processive end resection. The active site of RecJ in the N-terminal domain contains two divalent cations that coordinate the nucleophilic water. The ssDNA makes a 180° turn at the scissile phosphate. The C-terminal domain of RecJ binds the SSB-Ct, which explains how RecJ and SSB work together to efficiently process broken DNA ends for homologous recombination.