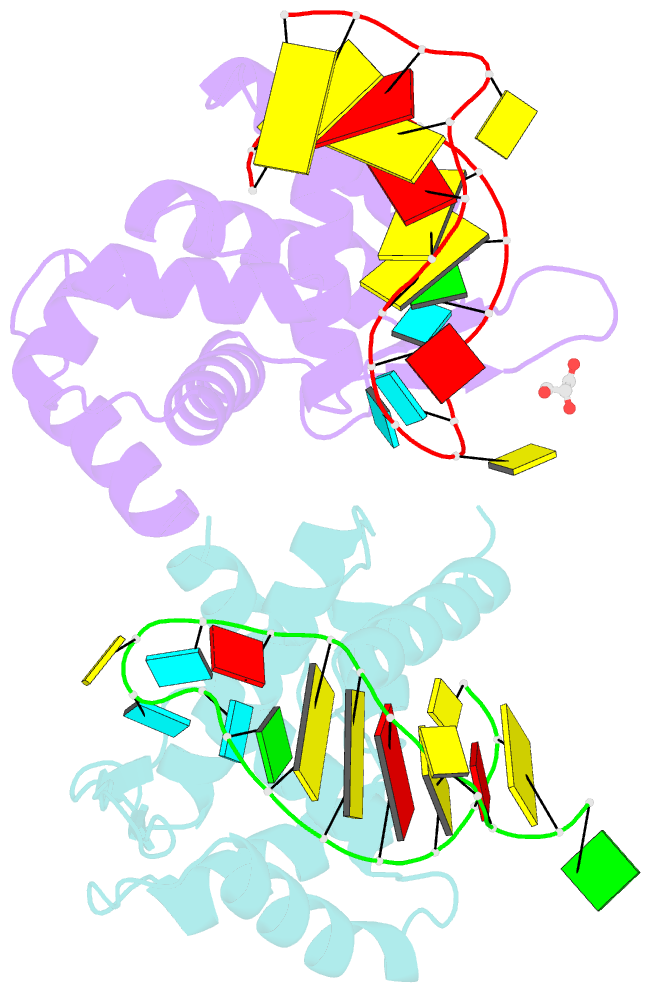
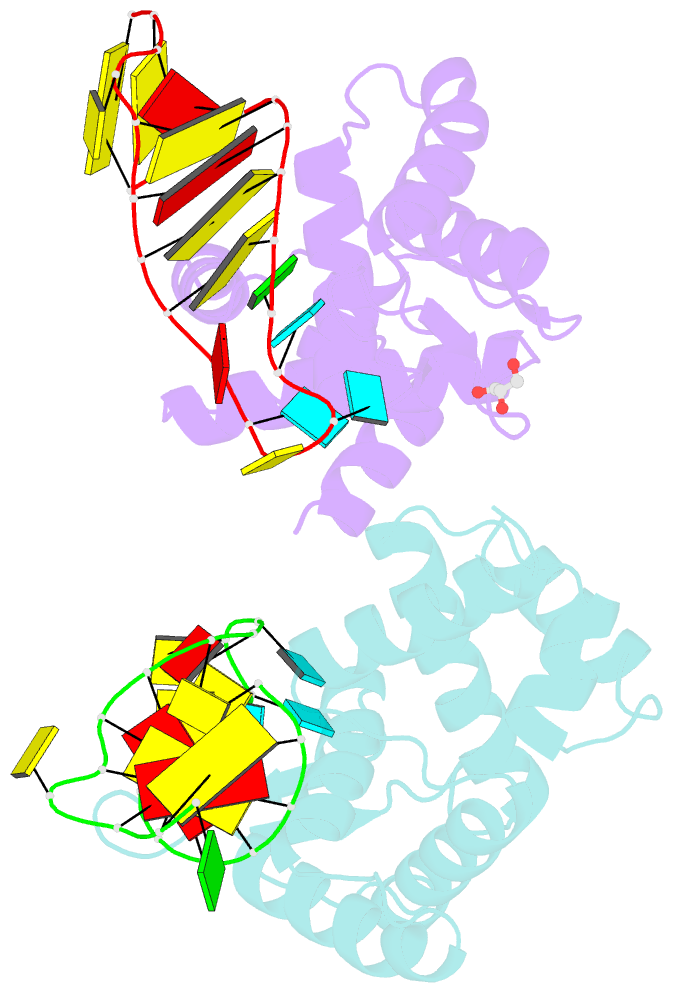
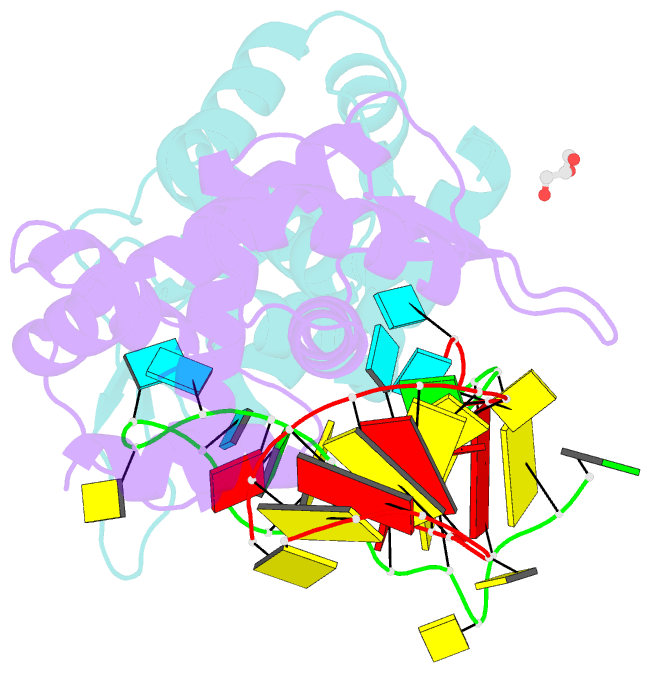
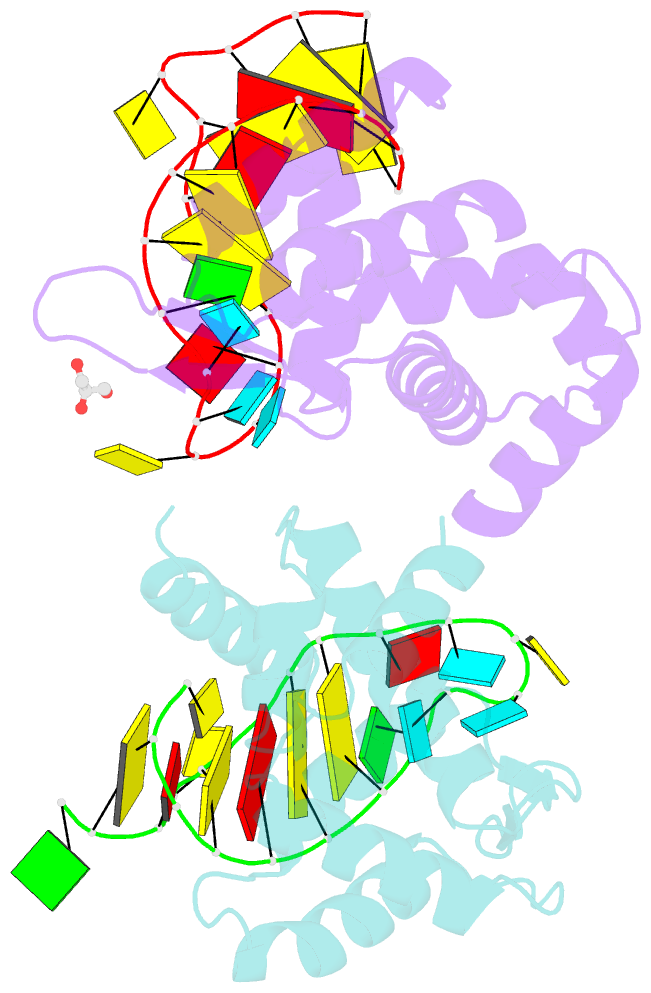
Summary information and primary citation
- PDB-id
- 5f5h; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein
- Method
- X-ray (2.23 Å)
- Summary
- X-ray structure of roquin roq domain in complex with ox40 hexa-loop RNA motif
- Reference
- Janowski R, Heinz GA, Schlundt A, Wommelsdorf N, Brenner S, Gruber AR, Blank M, Buch T, Buhmann R, Zavolan M, Niessing D, Heissmeyer V, Sattler M (2016): "Roquin recognizes a non-canonical hexaloop structure in the 3'-UTR of Ox40." Nat Commun, 7, 11032. doi: 10.1038/ncomms11032.
- Abstract
- The RNA-binding protein Roquin is required to prevent autoimmunity. Roquin controls T-helper cell activation and differentiation by limiting the induced expression of costimulatory receptors such as tumor necrosis factor receptor superfamily 4 (Tnfrs4 or Ox40). A constitutive decay element (CDE) with a characteristic triloop hairpin was previously shown to be recognized by Roquin. Here we use SELEX assays to identify a novel U-rich hexaloop motif, representing an alternative decay element (ADE). Crystal structures and NMR data show that the Roquin-1 ROQ domain recognizes hexaloops in the SELEX-derived ADE and in an ADE-like variant present in the Ox40 3'-UTR with identical binding modes. In cells, ADE-like and CDE-like motifs cooperate in the repression of Ox40 by Roquin. Our data reveal an unexpected recognition of hexaloop cis elements for the posttranscriptional regulation of target messenger RNAs by Roquin.