Summary information and primary citation
- PDB-id
- 5fdk; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (3.208 Å)
- Summary
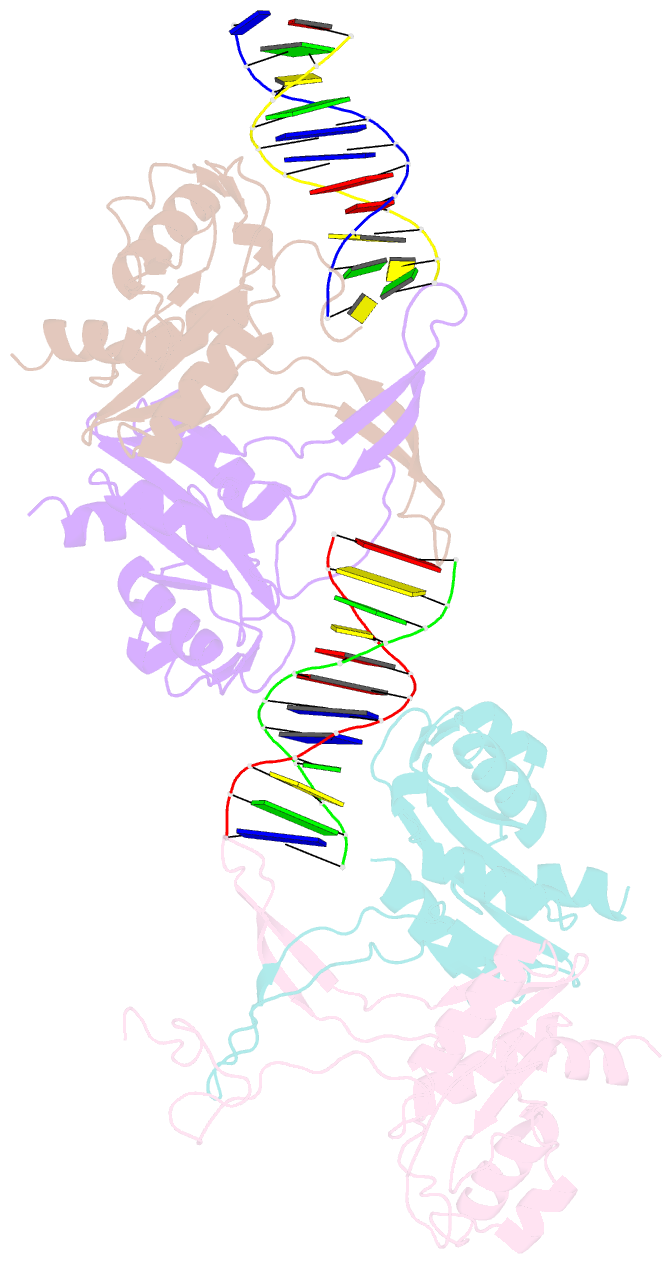
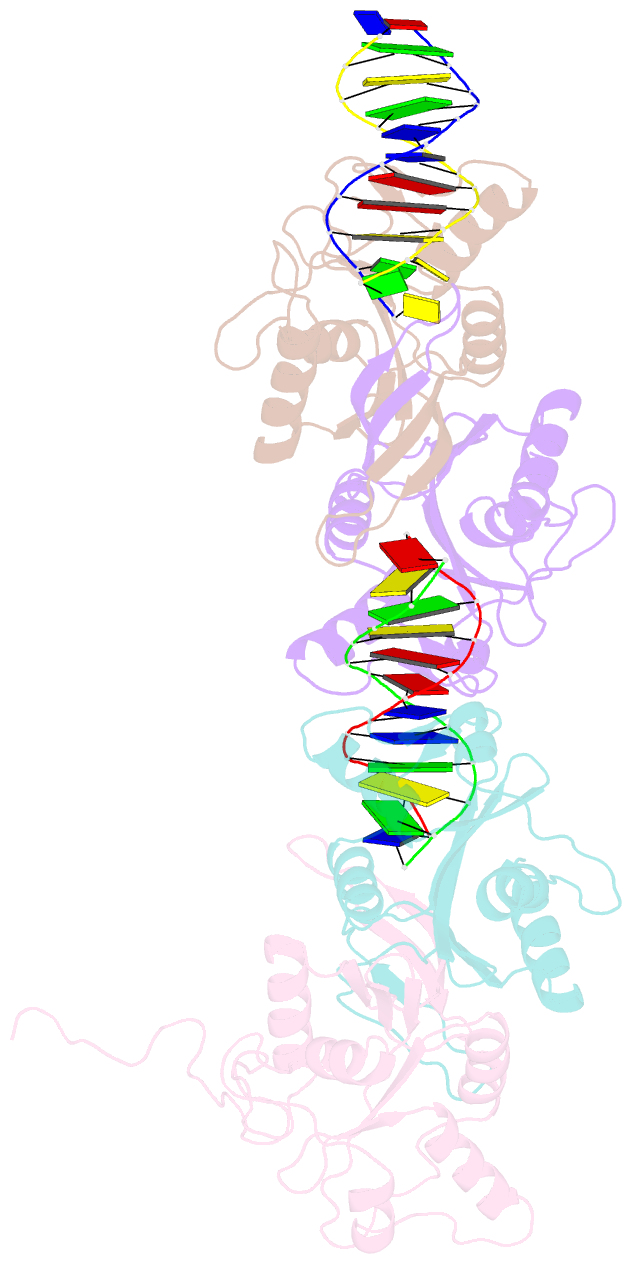
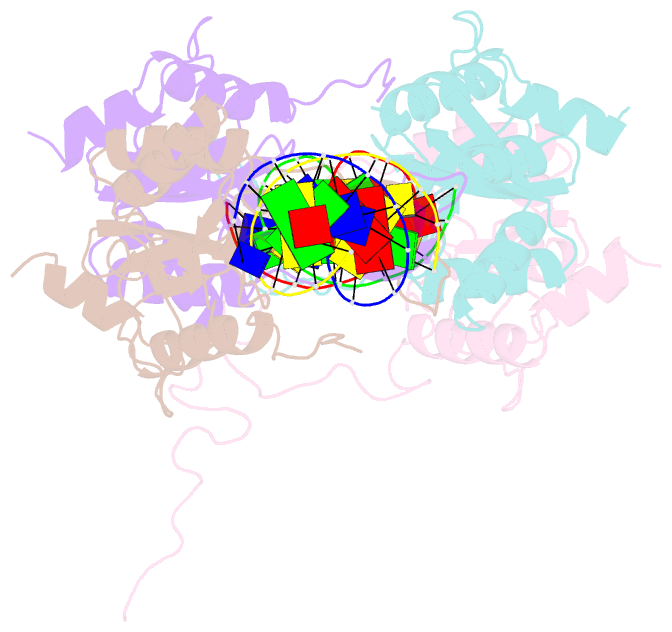
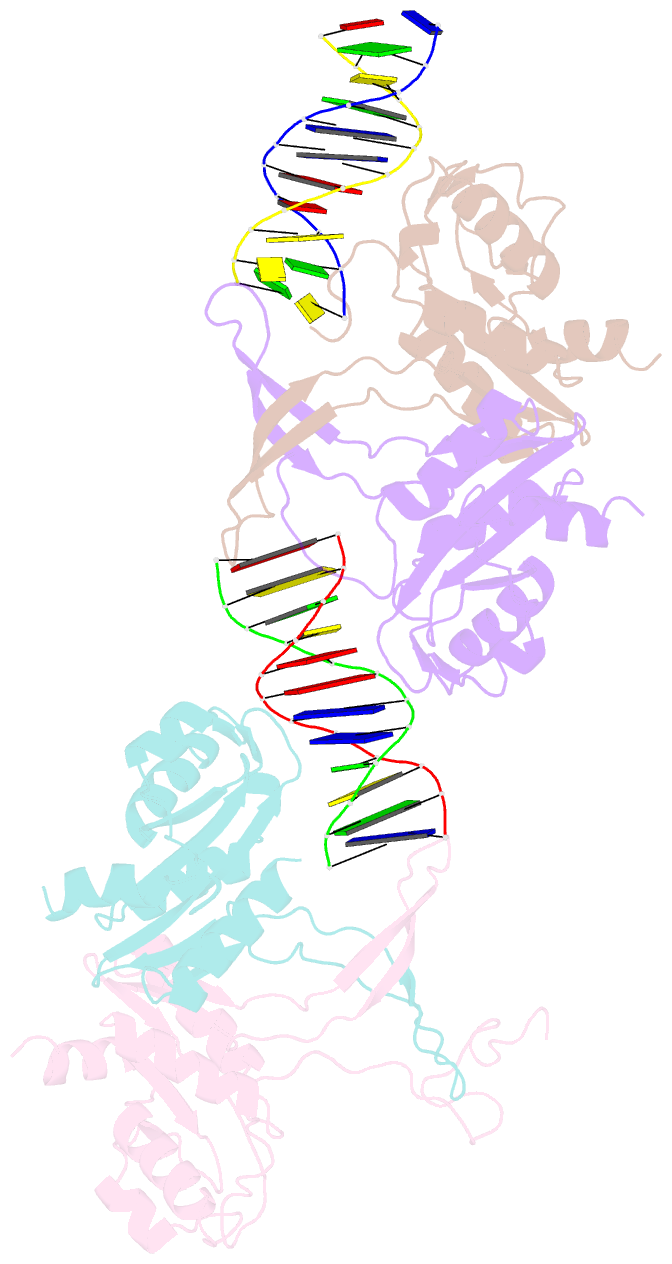
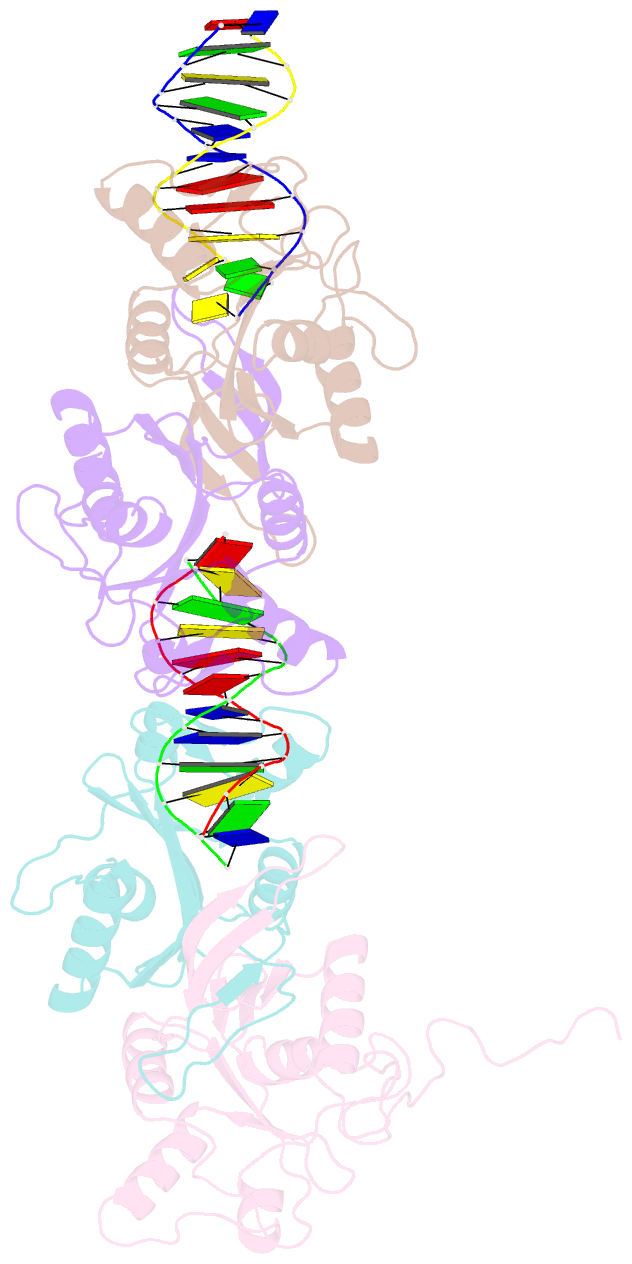
- Crystal structure of recu(d88n) in complex with palindromic DNA duplex
- Reference
- Khavnekar S, Dantu SC, Sedelnikova S, Ayora S, Rafferty J, Kale A (2017): "Structural insights into dynamics of RecU-HJ complex formation elucidates key role of NTR and stalk region toward formation of reactive state." Nucleic Acids Res., 45, 975-986. doi: 10.1093/nar/gkw1165.
- Abstract
- Holliday junction (HJ) resolving enzyme RecU is involved in DNA repair and recombination. We have determined the crystal structure of inactive mutant (D88N) of RecU from Bacillus subtilis in complex with a 12 base palindromic DNA fragment at a resolution of 3.2 Å. This structure shows the stalk region and the essential N-terminal region (NTR) previously unseen in our DNA unbound structure. The flexible nature of the NTR in solution was confirmed using SAXS. Thermofluor studies performed to assess the stability of RecU in complex with the arms of an HJ indicate that it confers stability. Further, we performed molecular dynamics (MD) simulations of wild type and an NTR deletion variant of RecU, with and without HJ. The NTR is observed to be highly flexible in simulations of the unbound RecU, in agreement with SAXS observations. These simulations revealed domain dynamics of RecU and their role in the formation of complex with HJ. The MD simulations also elucidate key roles of the NTR, stalk region, and breathing motion of RecU in the formation of the reactive state.