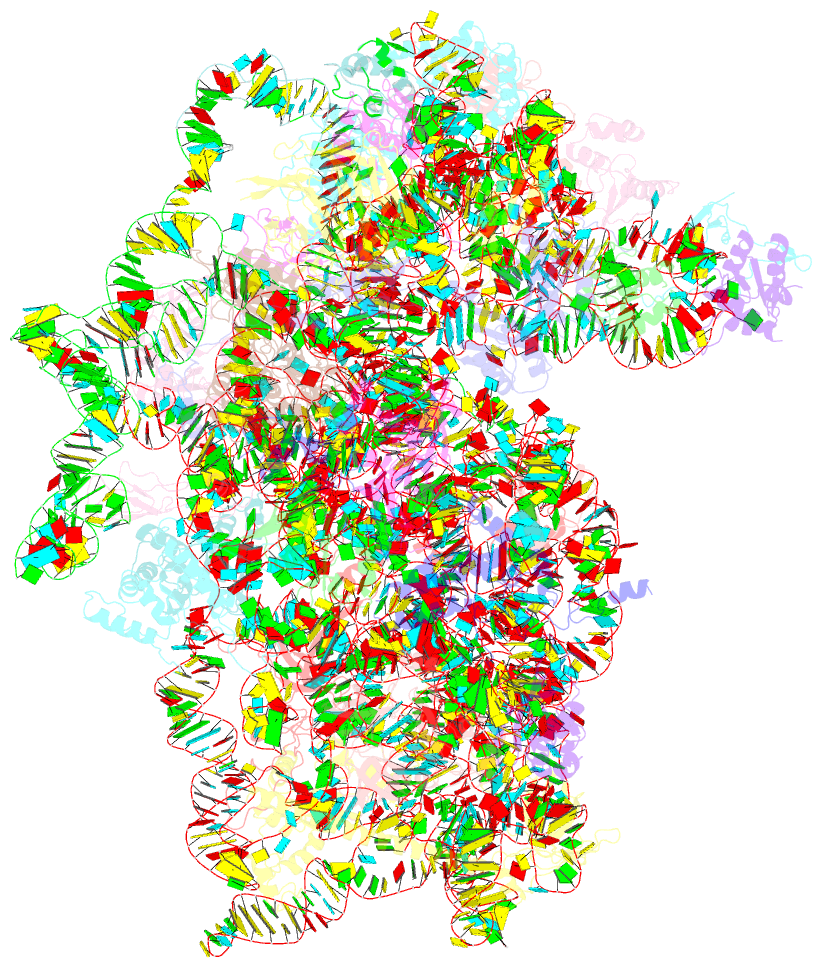
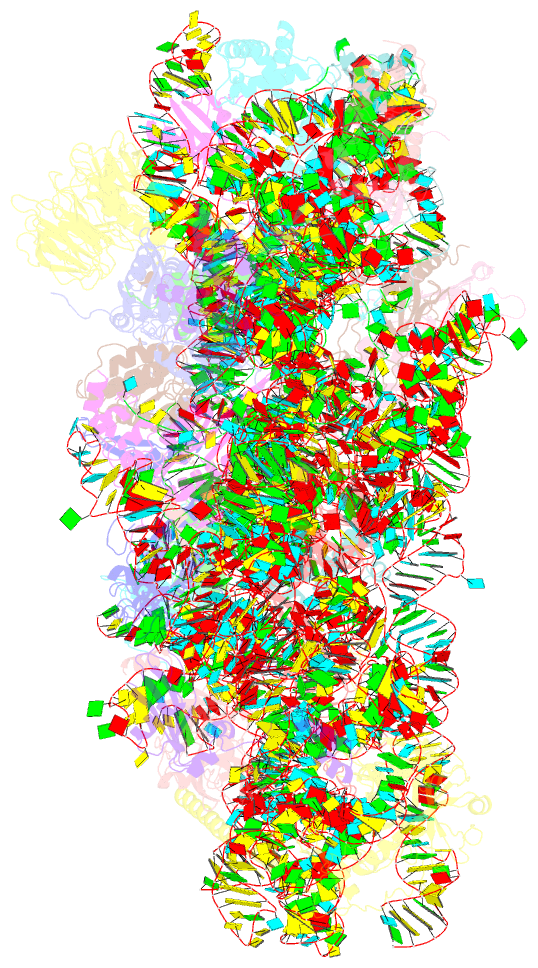
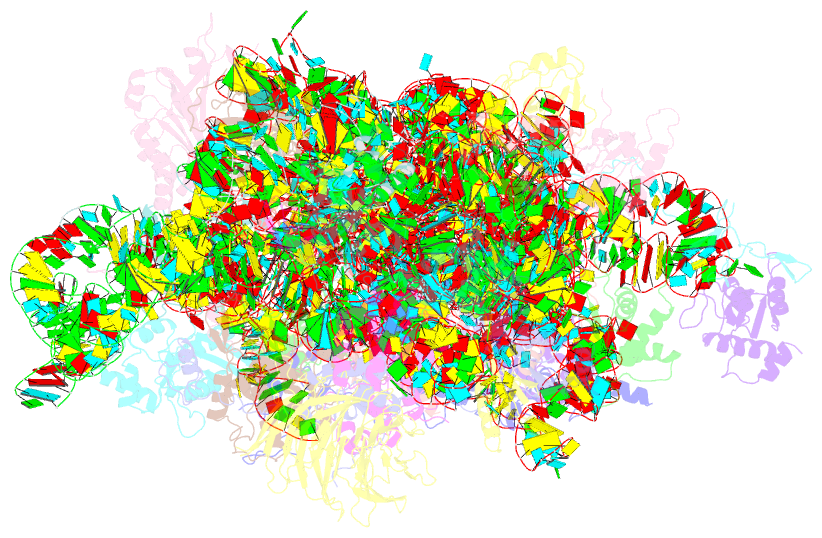
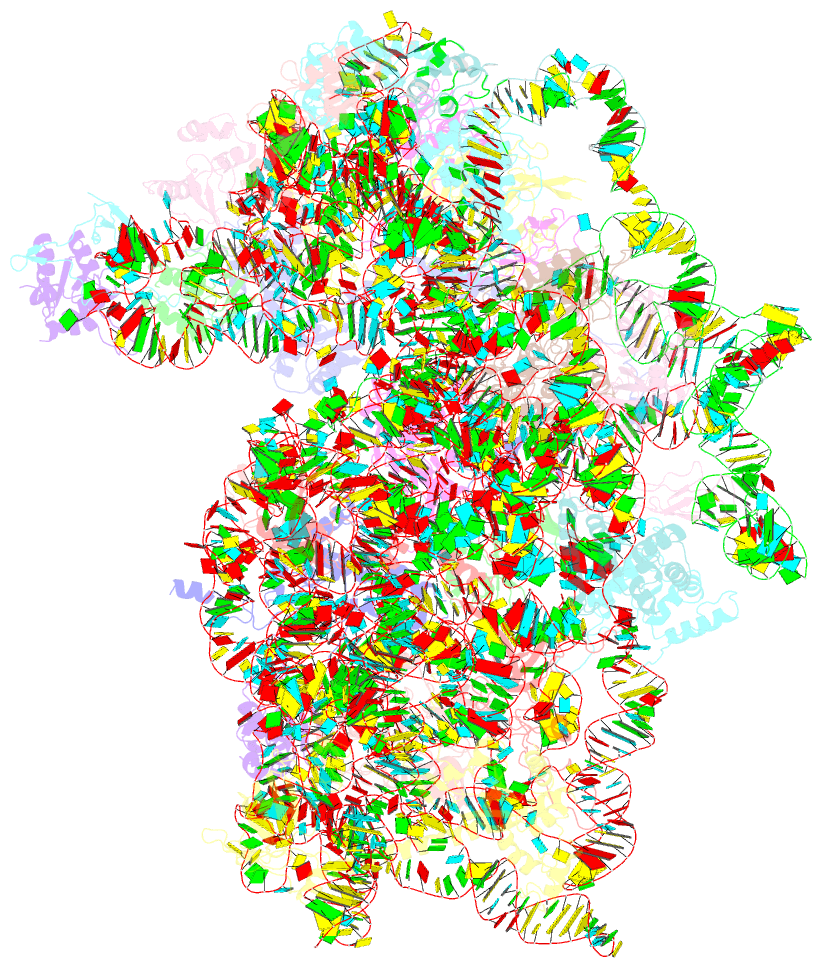
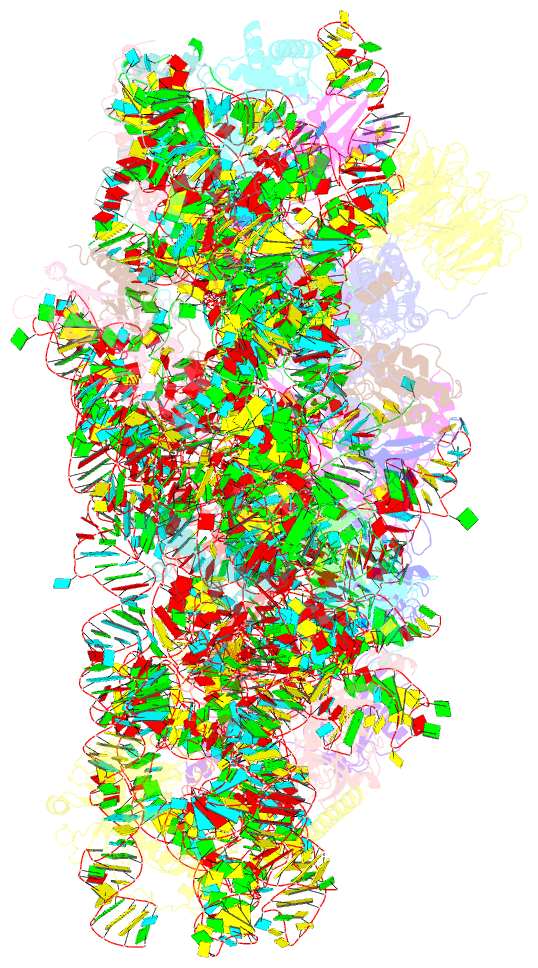
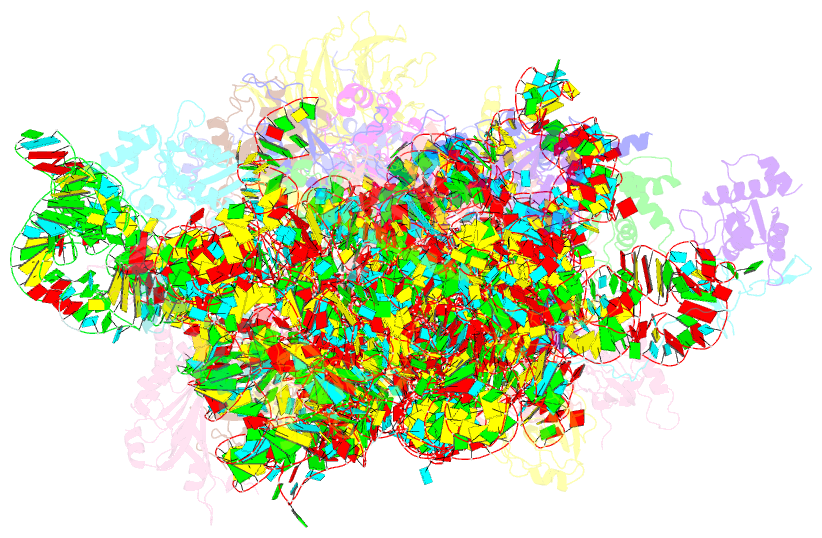
Summary information and primary citation
- PDB-id
- 5flx; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- ribosome
- Method
- cryo-EM (3.9 Å)
- Summary
- Mammalian 40s hcv-ires complex
- Reference
- Yamamoto H, Collier M, Loerke J, Ismer J, Schmidt A, Hilal T, Sprink T, Yamamoto K, Mielke T, Burger J, Shaikh TR, Dabrowski M, Hildebrand PW, Scheerer P, Spahn CM (2015): "Molecular Architecture of the Ribosome-Bound Hepatitis C Virus Internal Ribosomal Entry Site RNA." Embo J., 34, 3042. doi: 10.15252/EMBJ.201592469.
- Abstract
- Internal ribosomal entry sites (IRESs) are structured cis-acting RNAs that drive an alternative, cap-independent translation initiation pathway. They are used by many viruses to hijack the translational machinery of the host cell. IRESs facilitate translation initiation by recruiting and actively manipulating the eukaryotic ribosome using only a subset of canonical initiation factor and IRES transacting factors. Here we present cryo-EM reconstructions of the ribosome 80S- and 40S-bound Hepatitis C Virus (HCV) IRES. The presence of four subpopulations for the 80S•HCV IRES complex reveals dynamic conformational modes of the complex. At a global resolution of 3.9 Å for the most stable complex, a derived atomic model reveals a complex fold of the IRES RNA and molecular details of its interaction with the ribosome. The comparison of obtained structures explains how a modular architecture facilitates mRNA loading and tRNA binding to the P-site. This information provides the structural foundation for understanding the mechanism of HCV IRES RNA-driven translation initiation.