Summary information and primary citation
- PDB-id
- 5gkr; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- immune system-DNA
- Method
- X-ray (2.1 Å)
- Summary
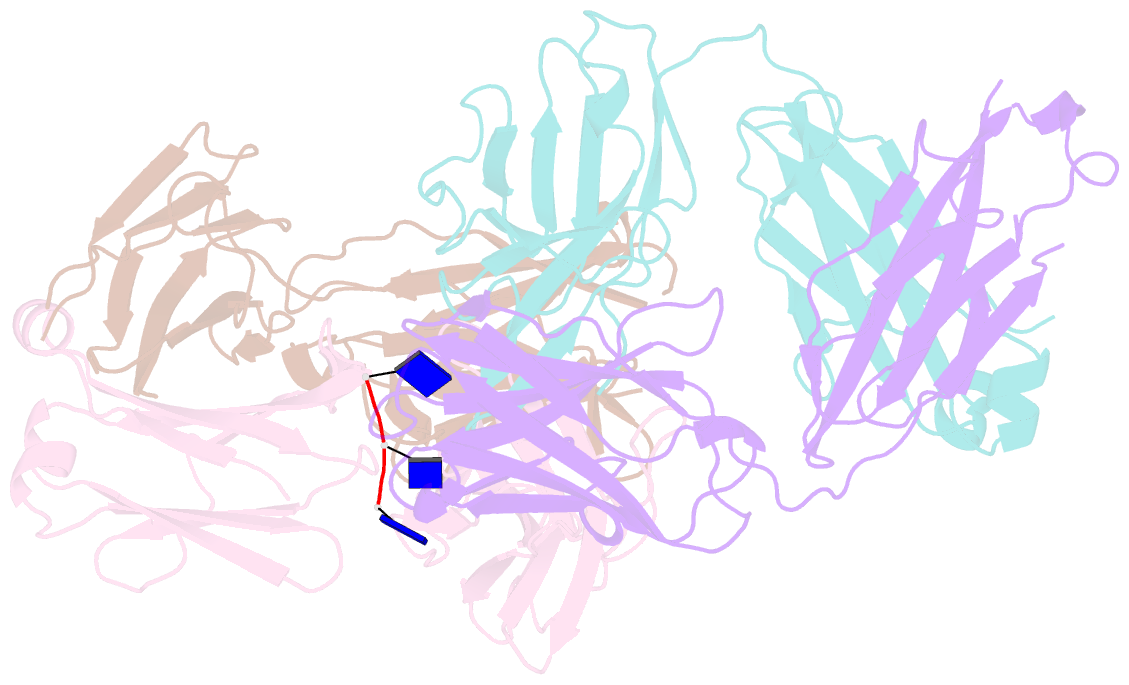
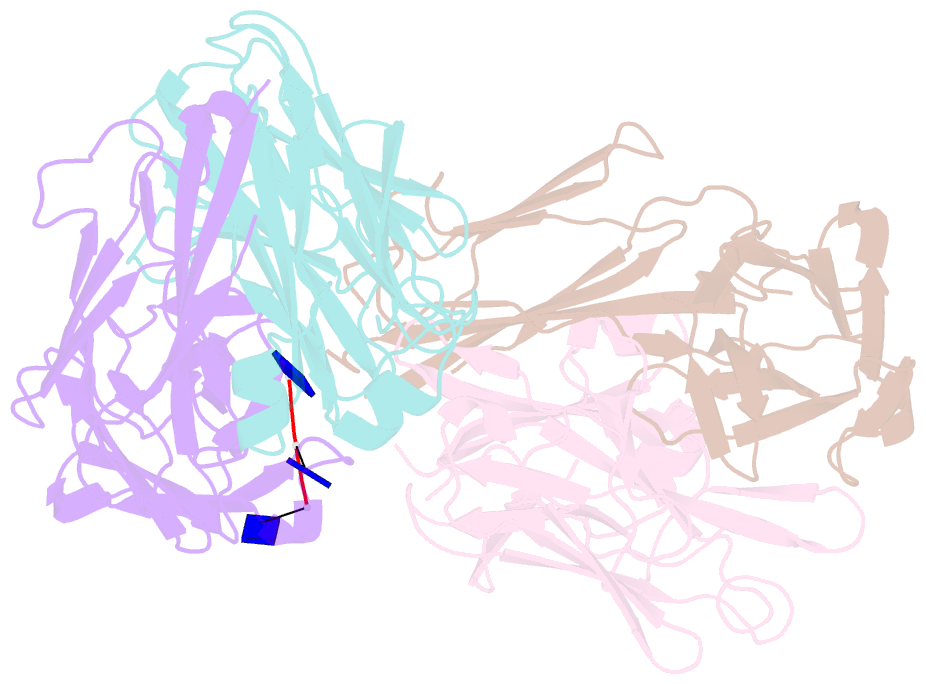
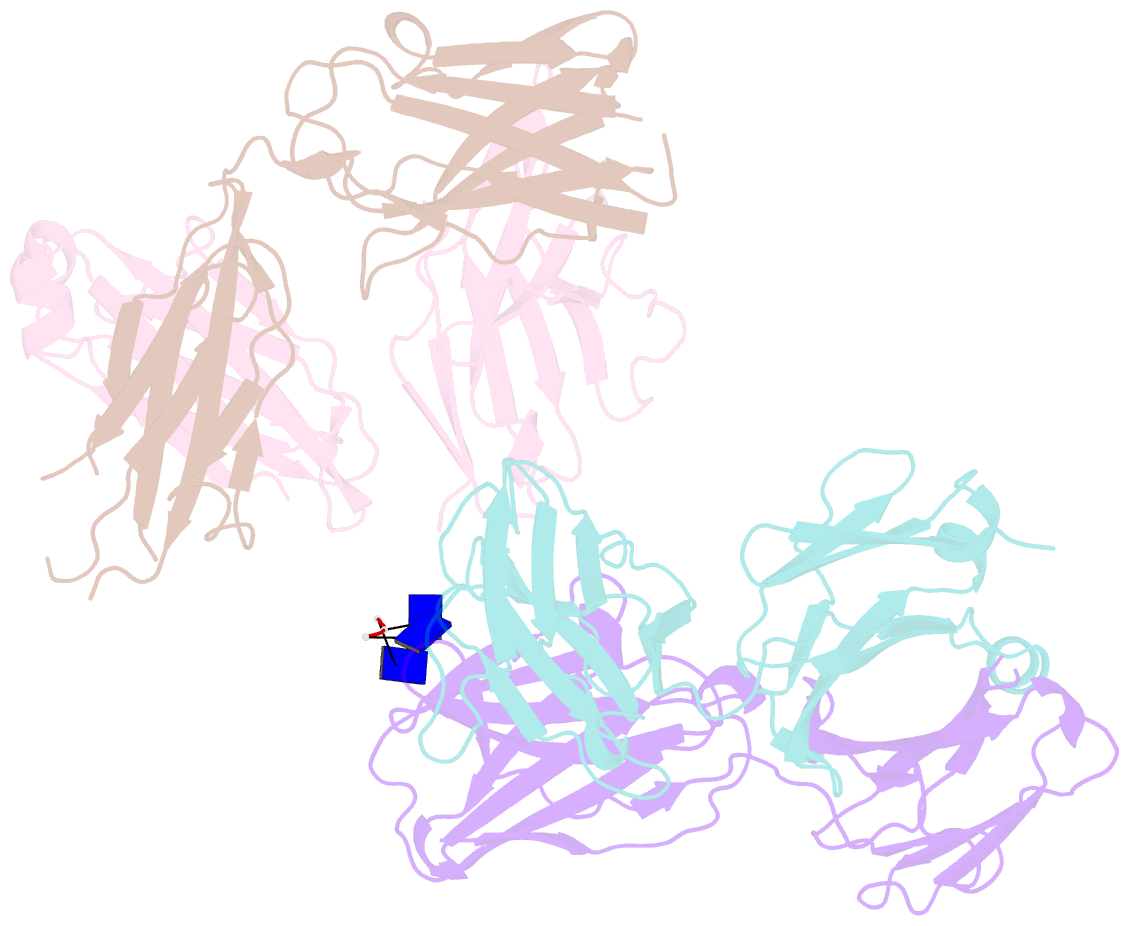
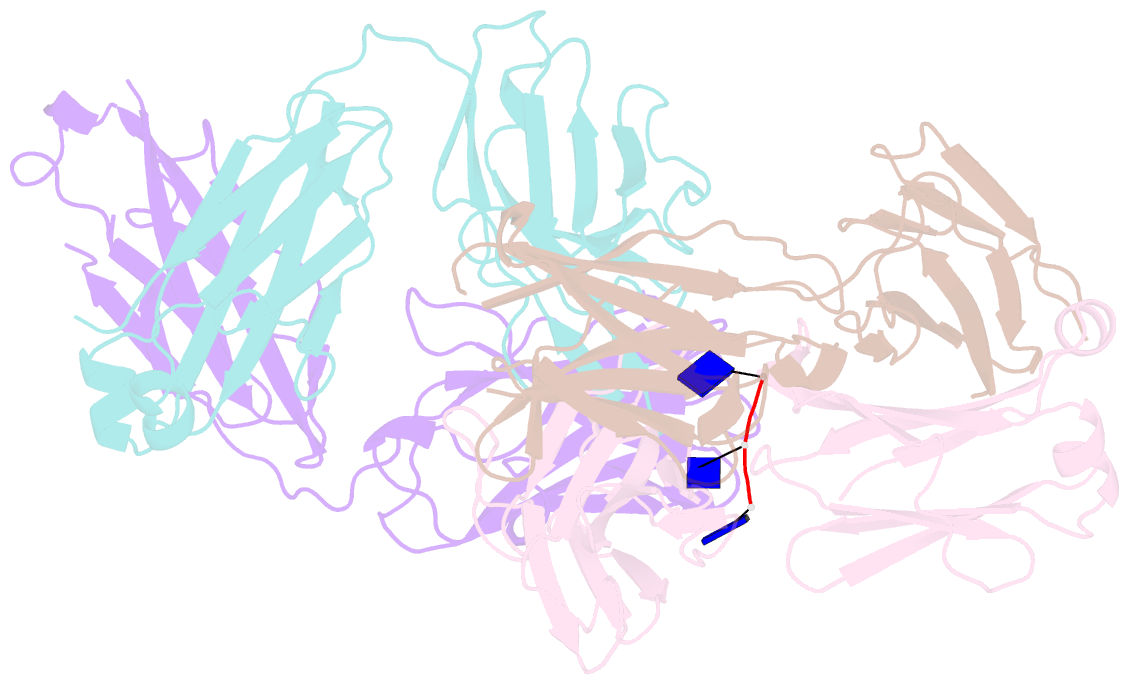
- Crystal structure of sle patient-derived anti-DNA antibody in complex with oligonucleotide
- Reference
- Sakakibara S, Arimori T, Yamashita K, Jinzai H, Motooka D, Nakamura S, Li S, Takeda K, Katayama J, El Hussien MA, Narazaki M, Tanaka T, Standley DM, Takagi J, Kikutani H (2017): "Clonal evolution and antigen recognition of anti-nuclear antibodies in acute systemic lupus erythematosus." Sci Rep, 7, 16428. doi: 10.1038/s41598-017-16681-y.
- Abstract
- The evolutional process of disease-associated autoantibodies in systemic lupus erythematosus (SLE) remains to be established. Here we show intraclonal diversification and affinity maturation of anti-nuclear antibody (ANA)-producing B cells in SLE. We identified a panel of monoclonal ANAs recognizing nuclear antigens, such as double-stranded DNA (dsDNA) and ribonucleoproteins (RNPs) from acute SLE subjects. These ANAs had relatively few, but nonetheless critical mutations. High-throughput immunoglobulin sequencing of blood lymphocytes disclosed the existence of sizable ANA lineages shearing critical mutations intraclonally. We further focused on anti-DNA antibodies, which are capable to bind to both single-stranded (ss) and dsDNA at high affinity. Crystal structure and biochemical analysis confirmed a direct role of the mutations in the acquisition of DNA reactivity and also revealed that these anti-DNA antibodies recognized an unpaired region within DNA duplex. Our study unveils the unique properties of high-affinity anti-DNA antibodies that are generated through antigen-driven affinity maturation in acute phase of SLE.