Summary information and primary citation
- PDB-id
- 5hnk; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase
- Method
- X-ray (2.22 Å)
- Summary
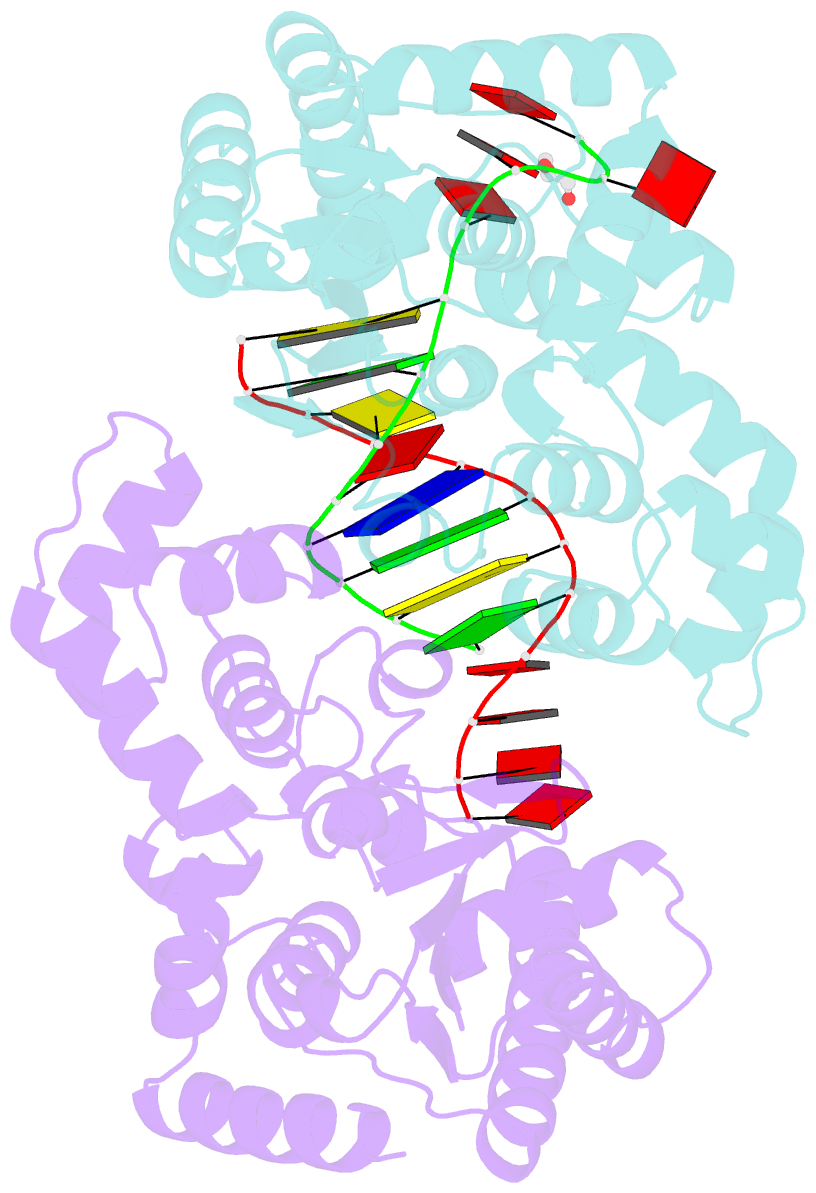
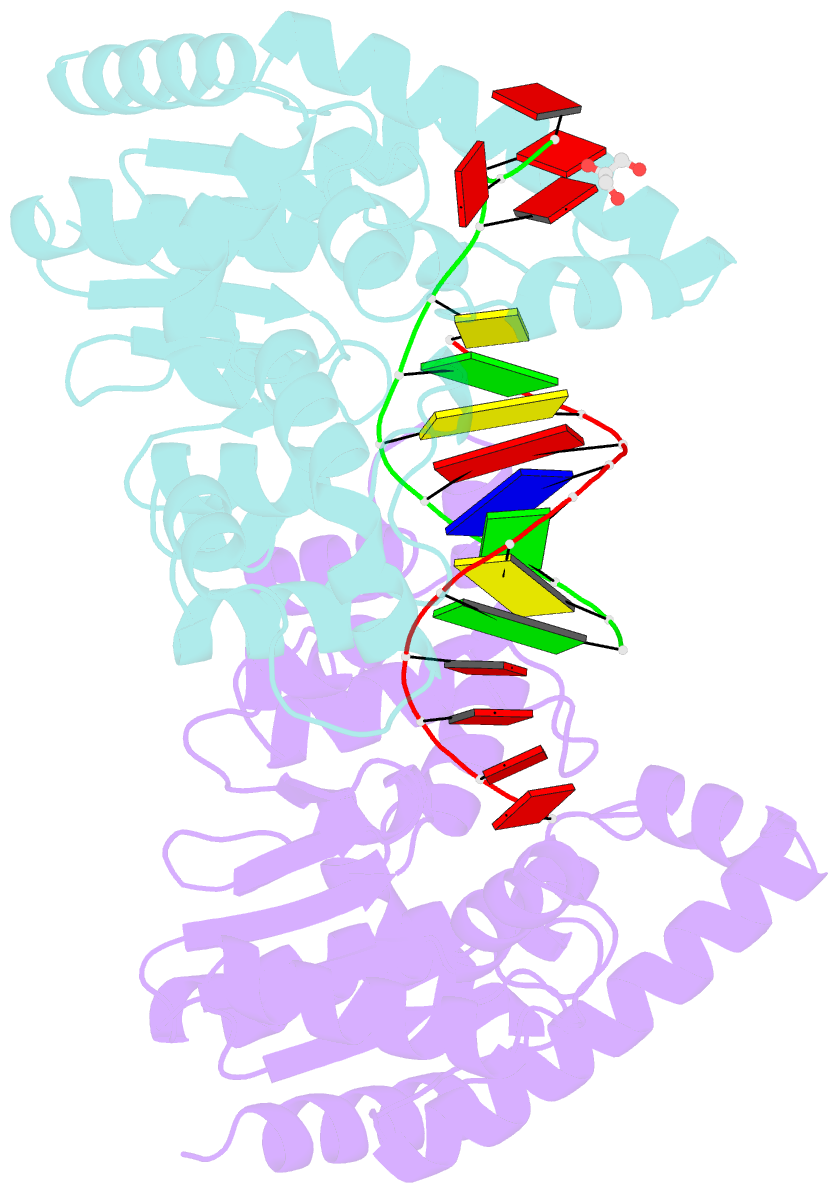
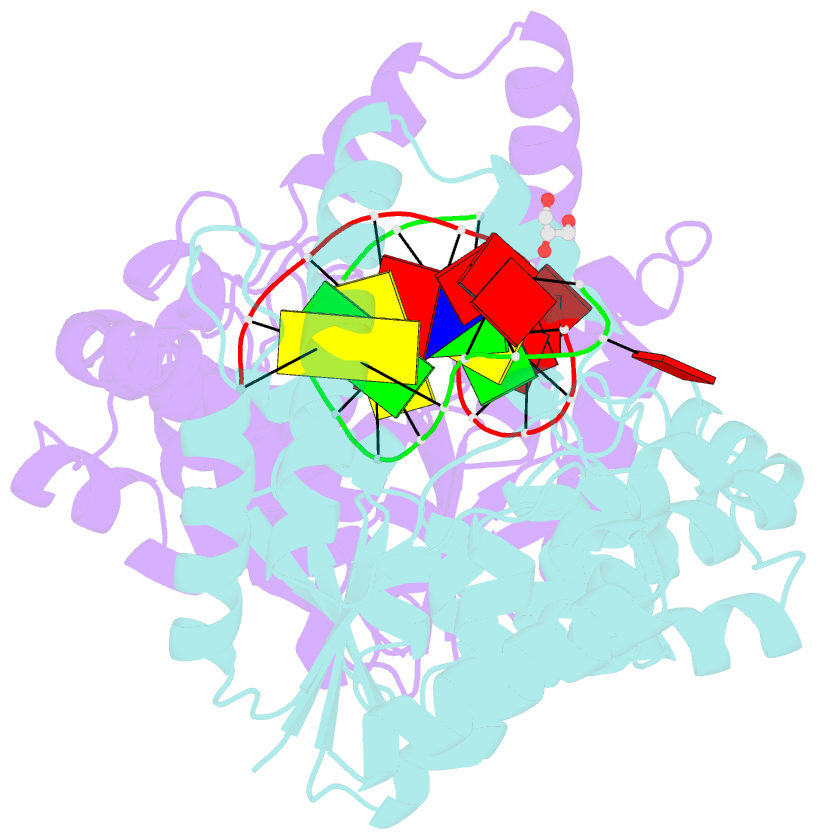
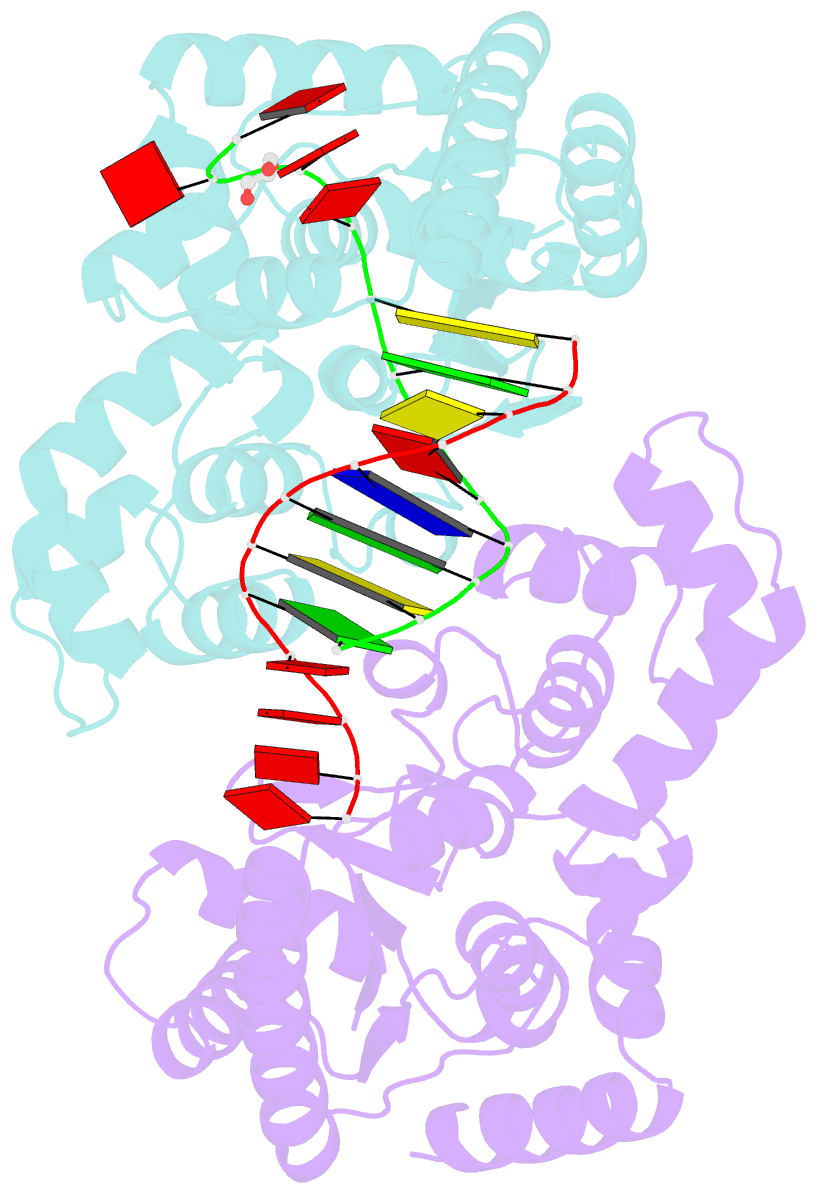
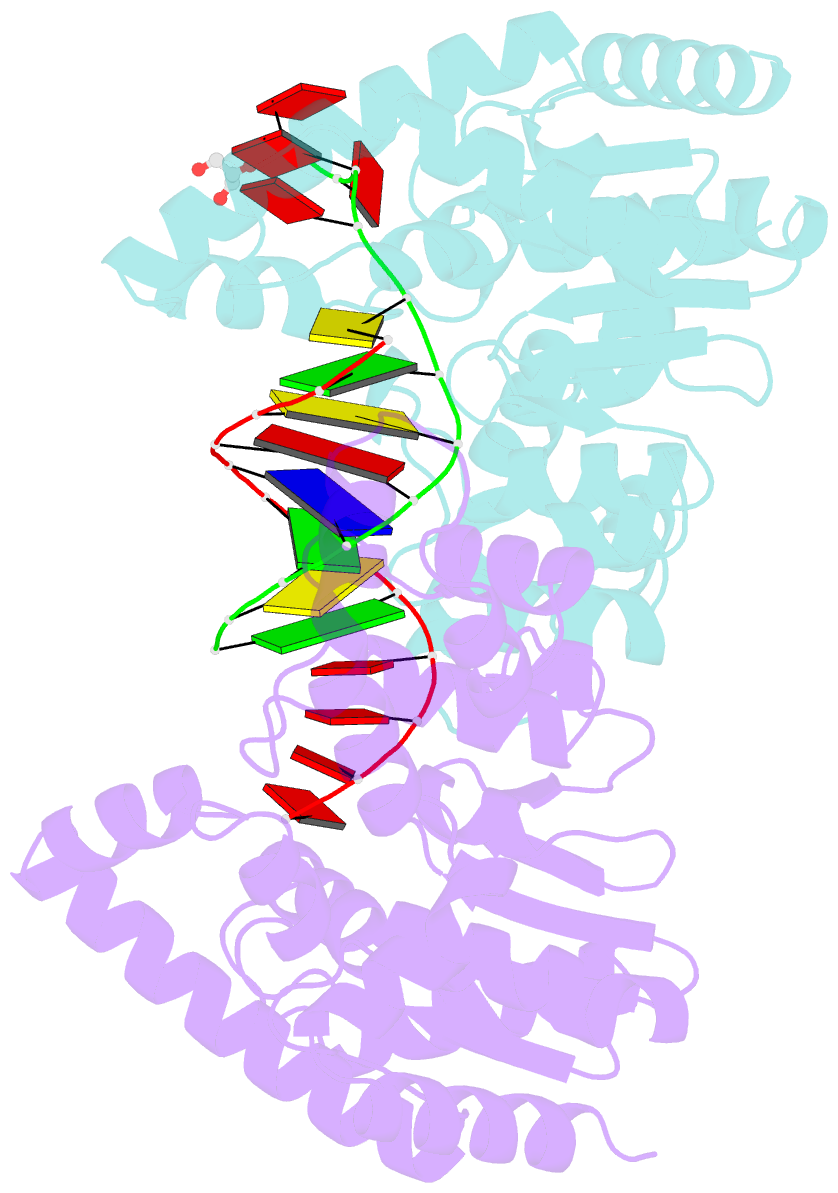
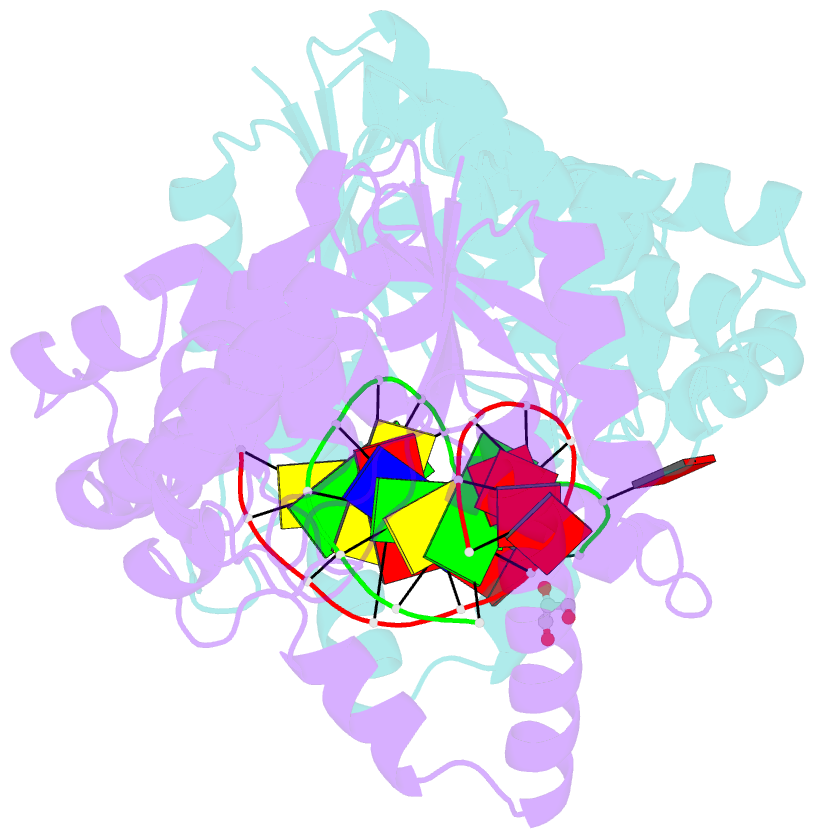
- Crystal structure of t5fen in complex intact substrate and metal ions.
- Reference
- AlMalki FA, Flemming CS, Zhang J, Feng M, Sedelnikova SE, Ceska T, Rafferty JB, Sayers JR, Artymiuk PJ (2016): "Direct observation of DNA threading in flap endonuclease complexes." Nat.Struct.Mol.Biol., 23, 640-646. doi: 10.1038/nsmb.3241.
- Abstract
- Maintenance of genome integrity requires that branched nucleic acid molecules be accurately processed to produce double-helical DNA. Flap endonucleases are essential enzymes that trim such branched molecules generated by Okazaki-fragment synthesis during replication. Here, we report crystal structures of bacteriophage T5 flap endonuclease in complexes with intact DNA substrates and products, at resolutions of 1.9-2.2 Å. They reveal single-stranded DNA threading through a hole in the enzyme, which is enclosed by an inverted V-shaped helical arch straddling the active site. Residues lining the hole induce an unusual barb-like conformation in the DNA substrate, thereby juxtaposing the scissile phosphate and essential catalytic metal ions. A series of complexes and biochemical analyses show how the substrate's single-stranded branch approaches, threads through and finally emerges on the far side of the enzyme. Our studies suggest that substrate recognition involves an unusual 'fly-casting, thread, bend and barb' mechanism.