Summary information and primary citation
- PDB-id
- 5hr7; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- oxidoreductase-RNA
- Method
- X-ray (2.4 Å)
- Summary
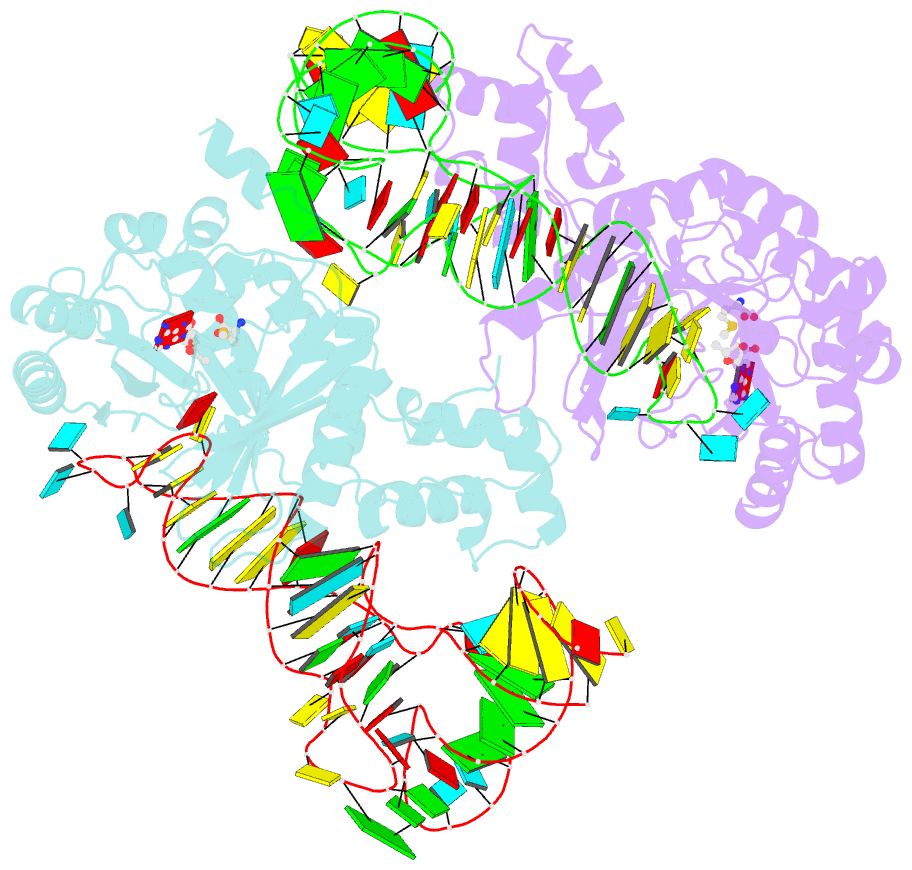
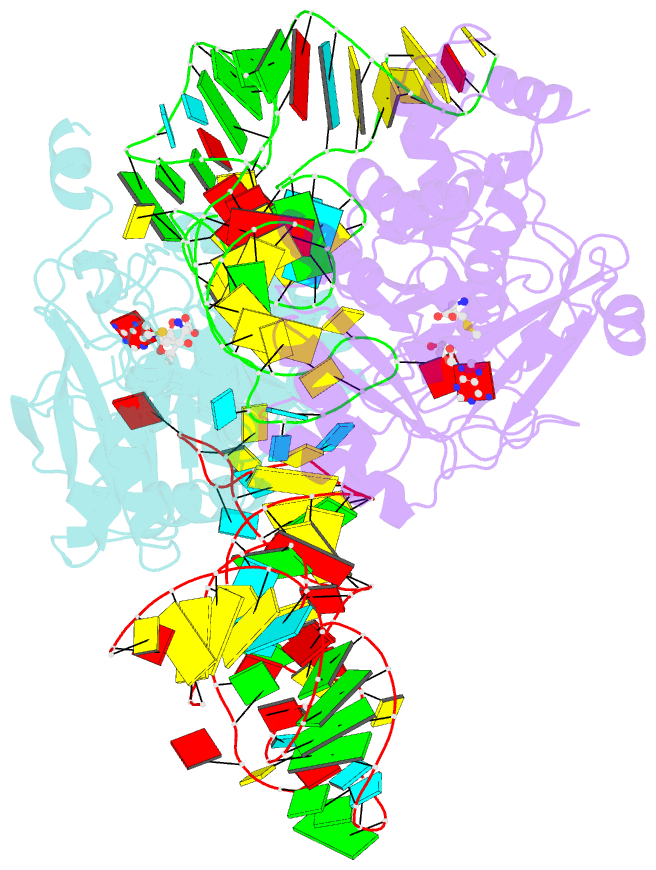
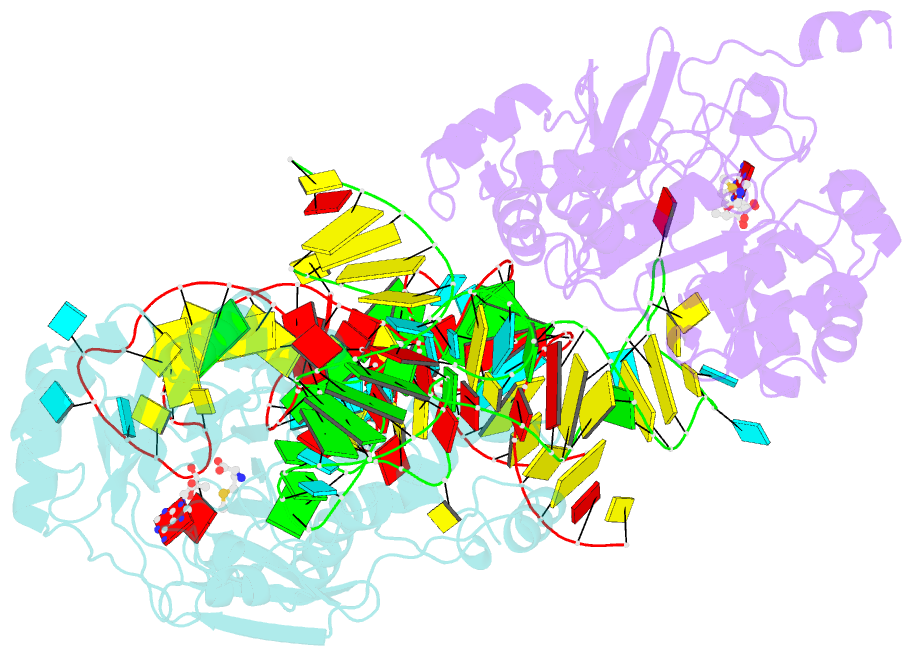
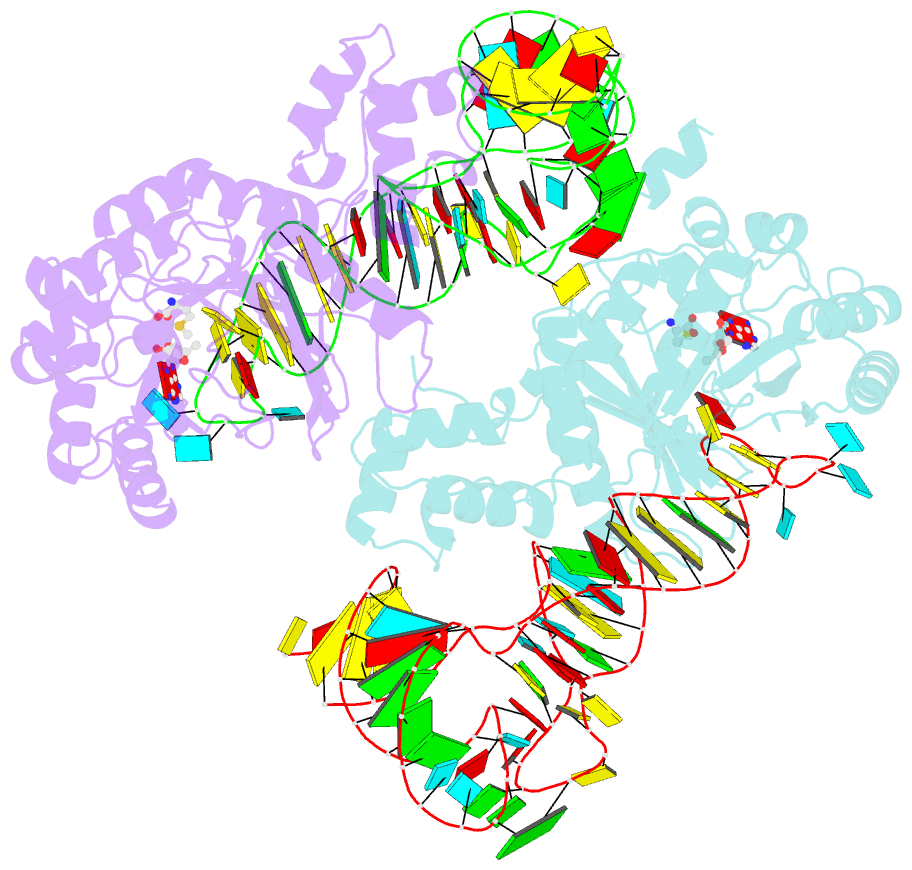
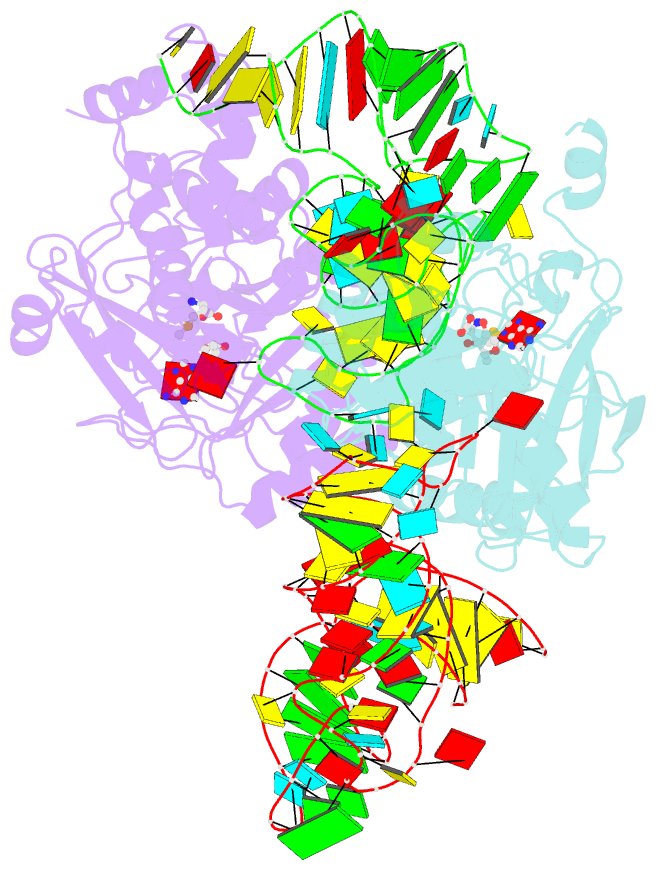
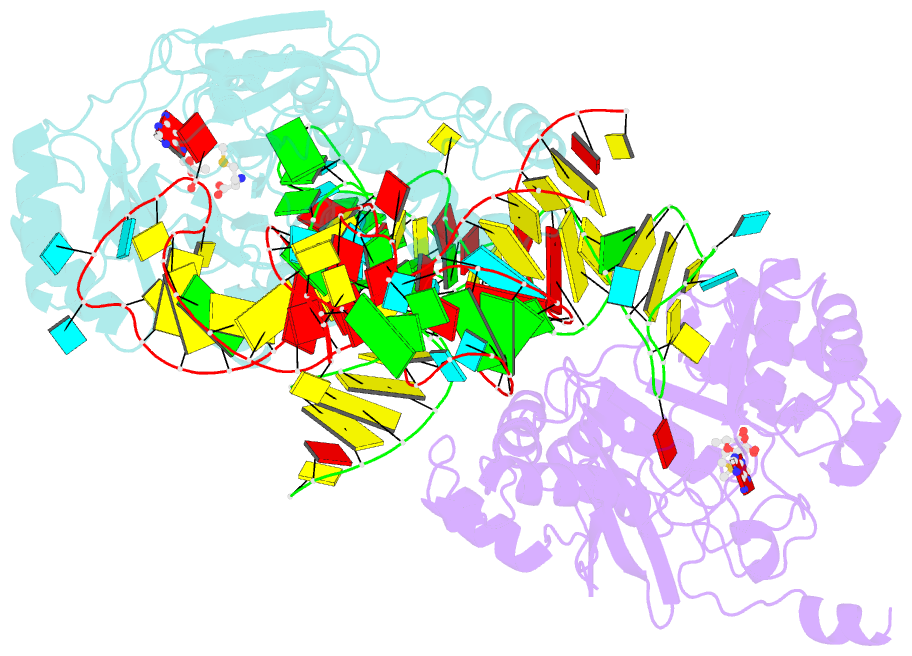
- X-ray crystal structure of c118a rlmn from escherichia coli with cross-linked in vitro transcribed trna
- Reference
- Schwalm EL, Grove TL, Booker SJ, Boal AK (2016): "Crystallographic capture of a radical S-adenosylmethionine enzyme in the act of modifying tRNA." Science, 352, 309-312. doi: 10.1126/science.aad5367.
- Abstract
- RlmN is a dual-specificity RNA methylase that modifies C2 of adenosine 2503 (A2503) in 23S rRNA and C2 of adenosine 37 (A37) in several Escherichia coli transfer RNAs (tRNAs). A related methylase, Cfr, modifies C8 of A2503 via a similar mechanism, conferring resistance to multiple classes of antibiotics. Here, we report the x-ray structure of a key intermediate in the RlmN reaction, in which a Cys(118)→Ala variant of the protein is cross-linked to a tRNA(Glu)substrate through the terminal methylene carbon of a formerly methylcysteinyl residue and C2 of A37. RlmN contacts the entire length of tRNA(Glu), accessing A37 by using an induced-fit strategy that completely unfolds the tRNA anticodon stem-loop, which is likely critical for recognition of both tRNA and ribosomal RNA substrates.