Summary information and primary citation
- PDB-id
- 5i4a; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- RNA binding protein-RNA
- Method
- X-ray (1.949 Å)
- Summary
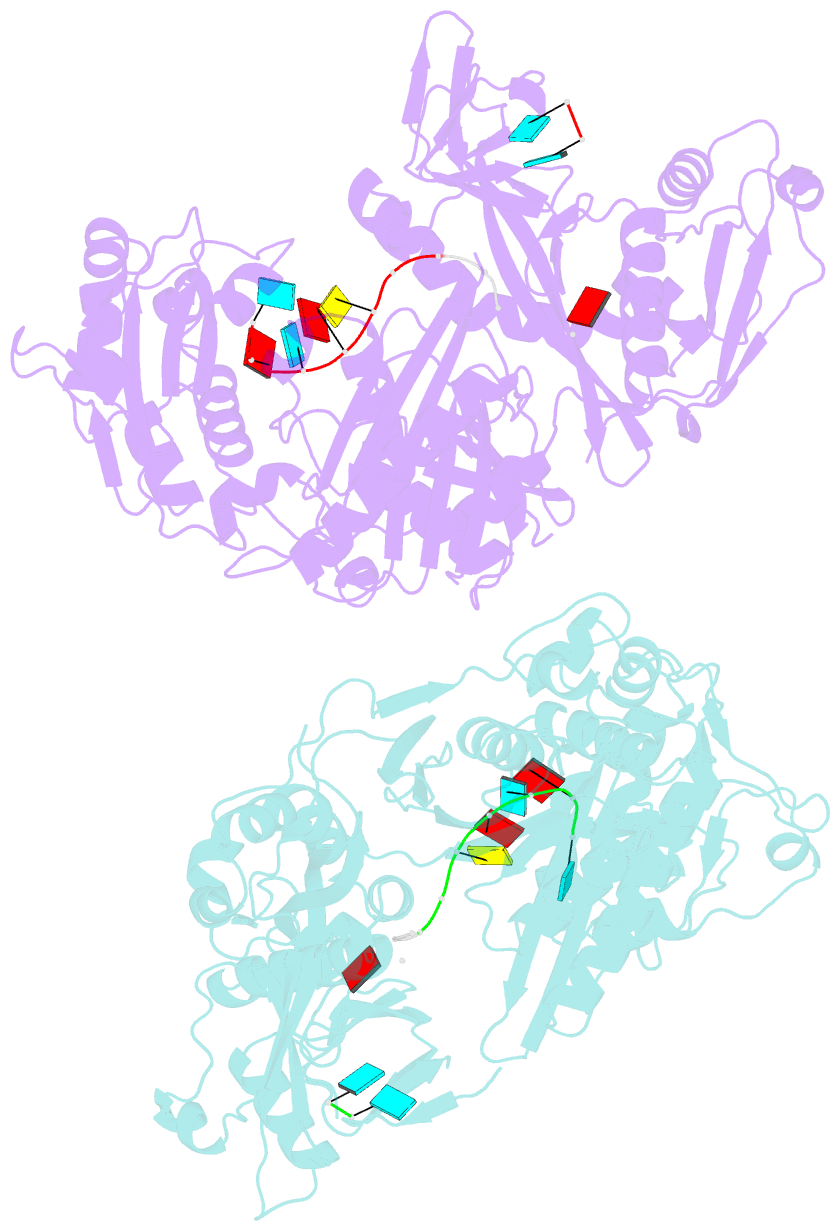
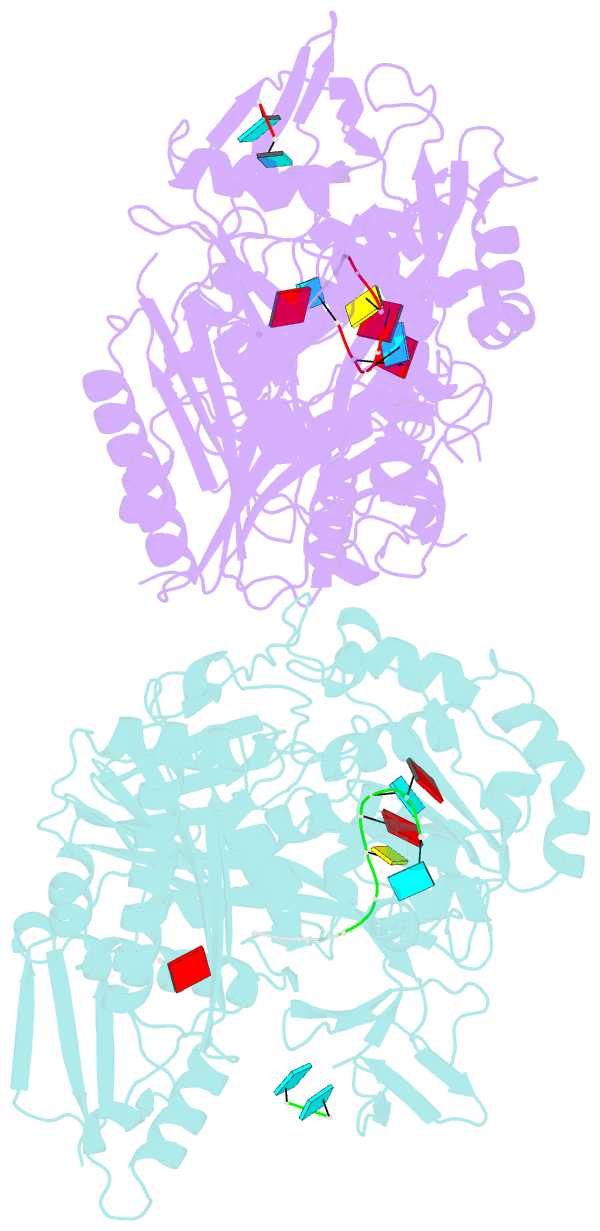
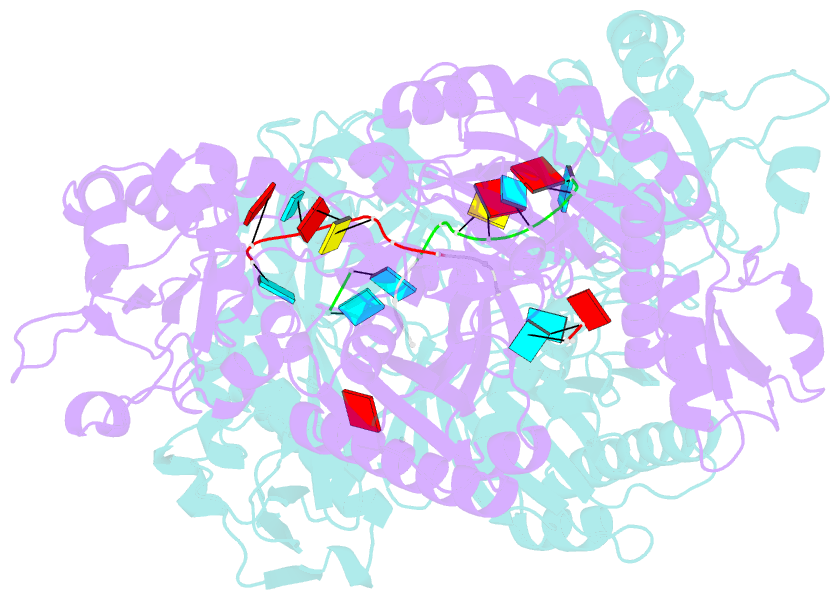
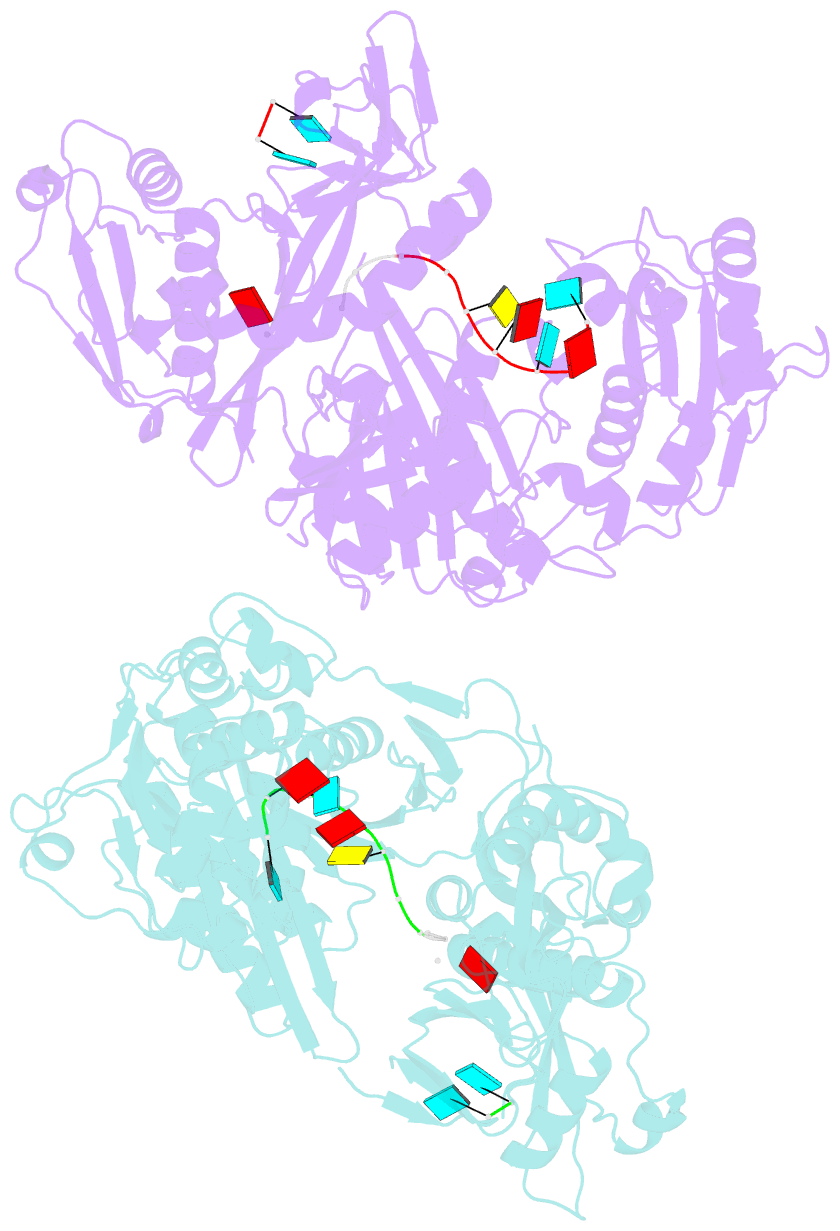
- X-ray crystal structure of marinitoga piezophila argonaute in complex with 5' oh guide RNA
- Reference
- Kaya E, Doxzen KW, Knoll KR, Wilson RC, Strutt SC, Kranzusch PJ, Doudna JA (2016): "A bacterial Argonaute with noncanonical guide RNA specificity." Proc.Natl.Acad.Sci.USA, 113, 4057-4062. doi: 10.1073/pnas.1524385113.
- Abstract
- Eukaryotic Argonaute proteins induce gene silencing by small RNA-guided recognition and cleavage of mRNA targets. Although structural similarities between human and prokaryotic Argonautes are consistent with shared mechanistic properties, sequence and structure-based alignments suggested that Argonautes encoded within CRISPR-cas [clustered regularly interspaced short palindromic repeats (CRISPR)-associated] bacterial immunity operons have divergent activities. We show here that the CRISPR-associated Marinitoga piezophila Argonaute (MpAgo) protein cleaves single-stranded target sequences using 5'-hydroxylated guide RNAs rather than the 5'-phosphorylated guides used by all known Argonautes. The 2.0-Å resolution crystal structure of an MpAgo-RNA complex reveals a guide strand binding site comprising residues that block 5' phosphate interactions. Using structure-based sequence alignment, we were able to identify other putative MpAgo-like proteins, all of which are encoded within CRISPR-cas loci. Taken together, our data suggest the evolution of an Argonaute subclass with noncanonical specificity for a 5'-hydroxylated guide.