Summary information and primary citation
- PDB-id
- 5inp; SNAP-derived features in text and JSON formats;
DNAproDB
- Class
- hydrolase-DNA
- Method
- X-ray (1.947 Å)
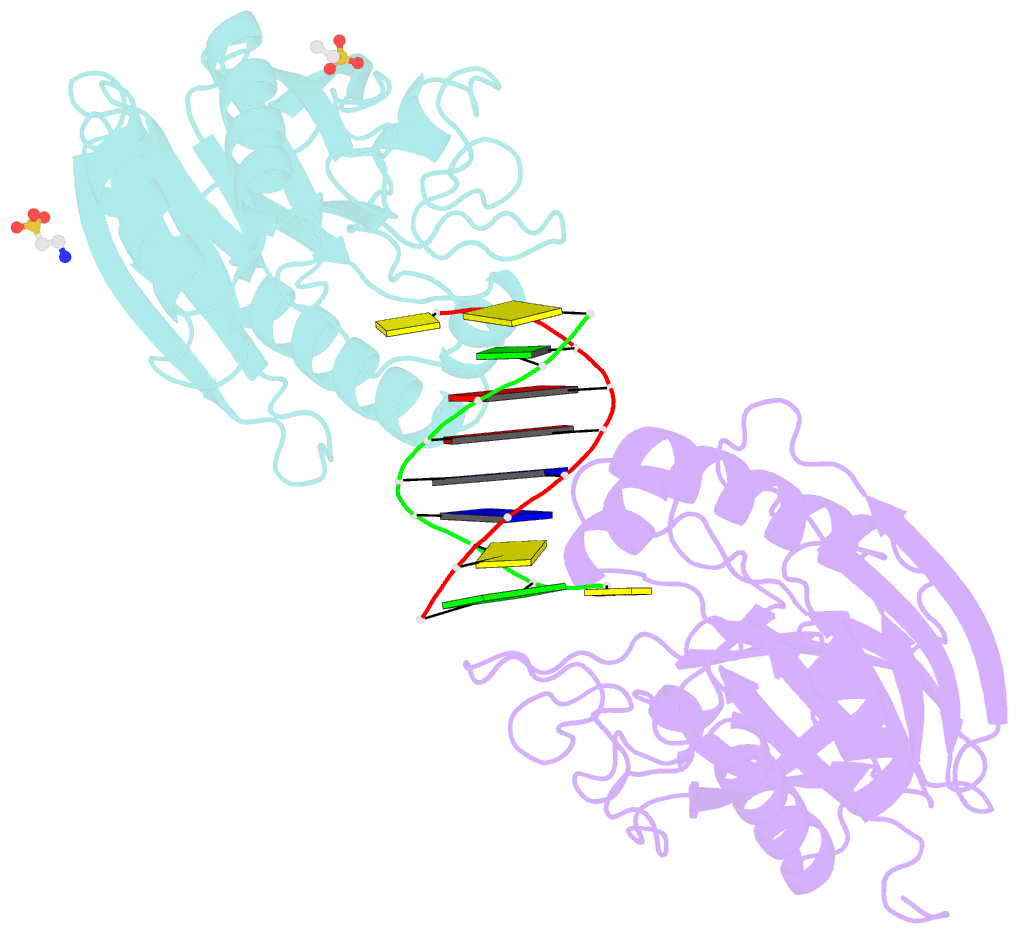
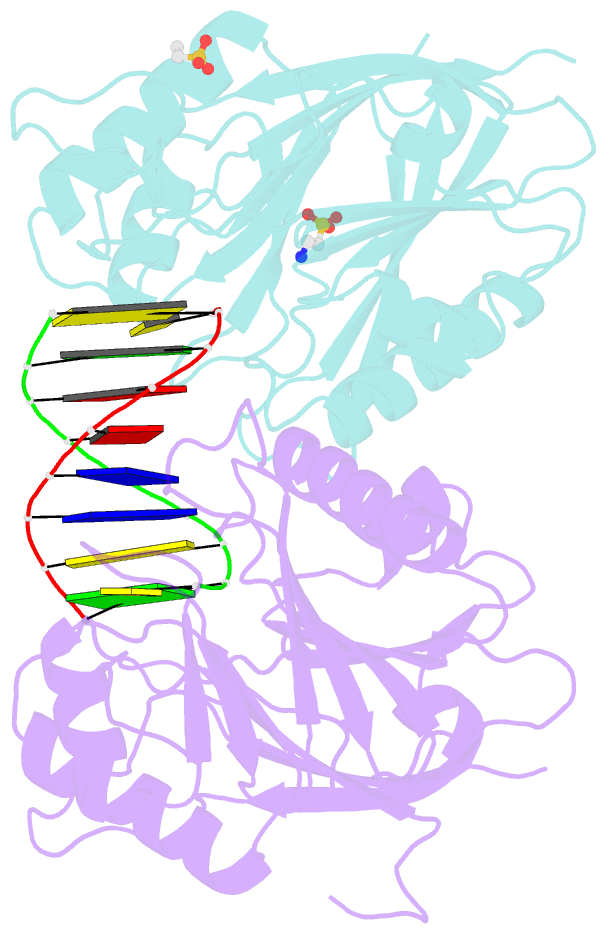
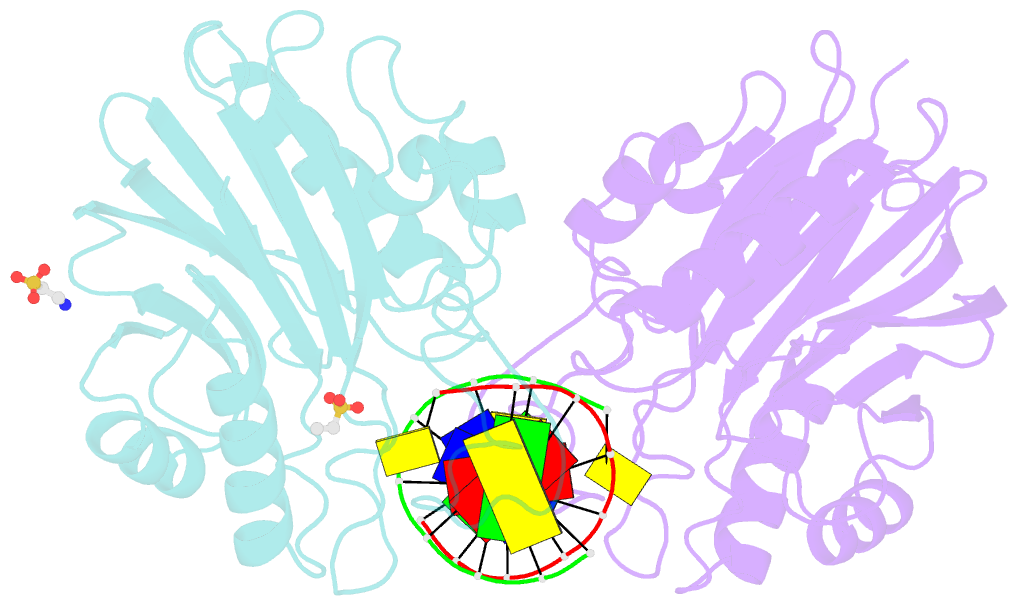
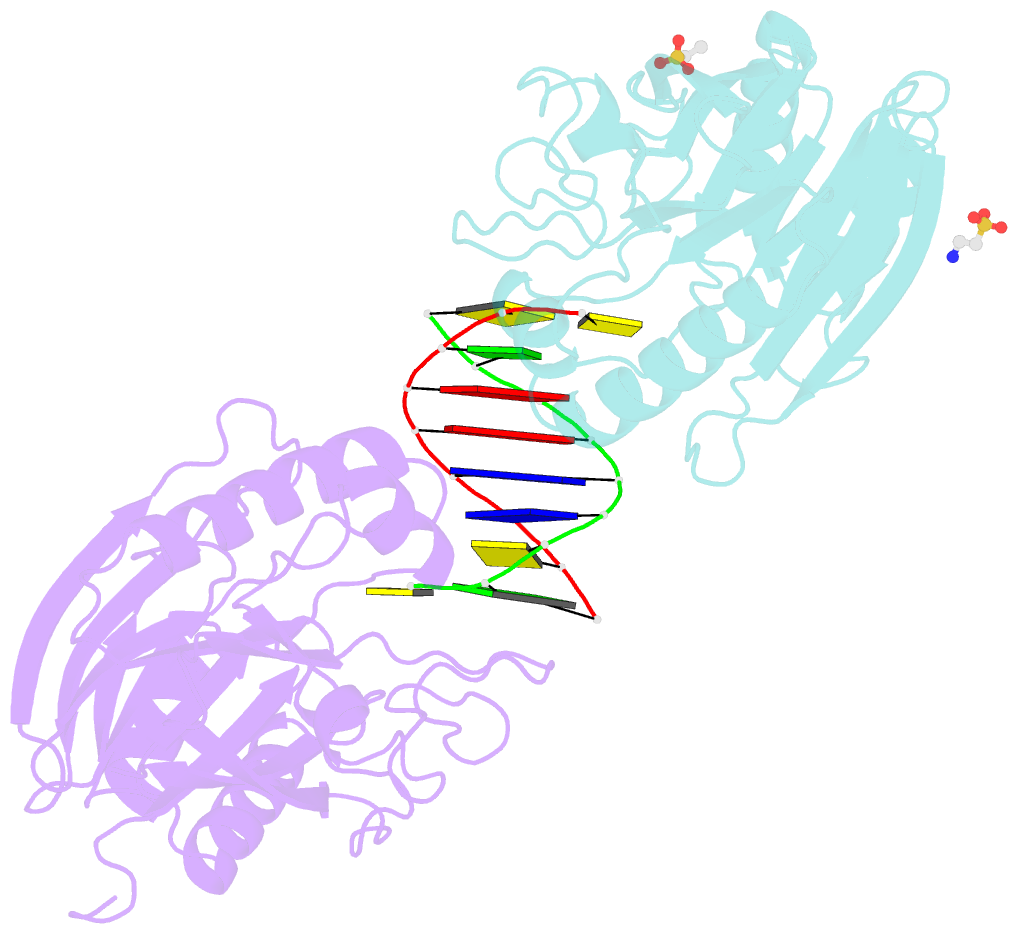
- Summary
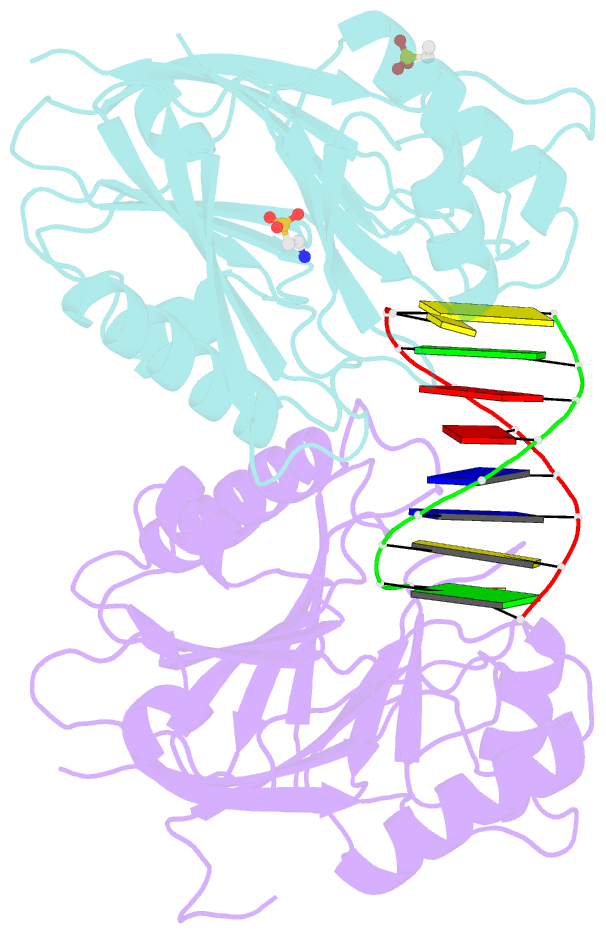
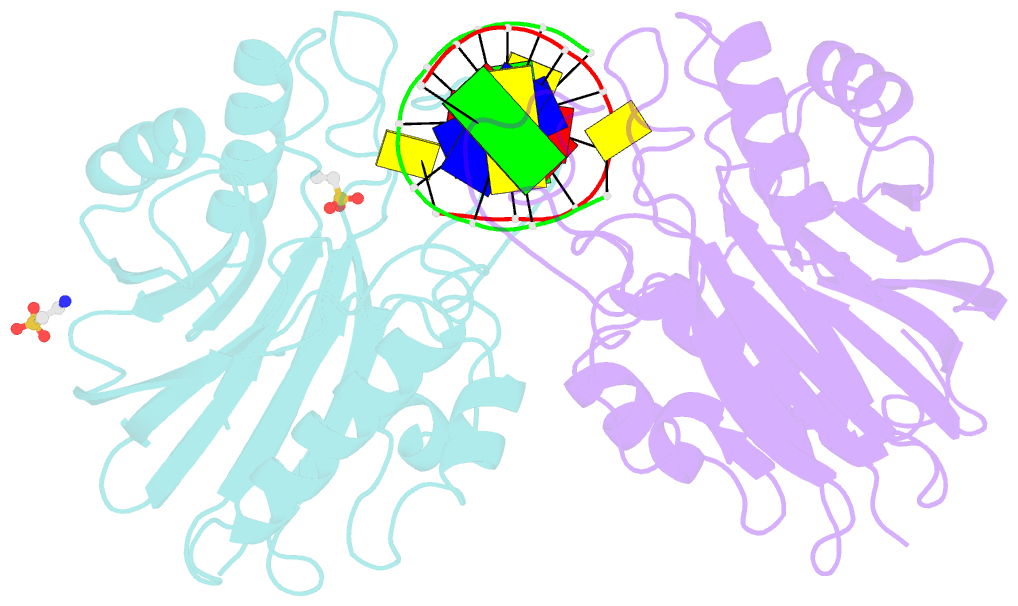
- Mouse tdp2 reaction product (5'-phosphorylated DNA)-mn2+ complex
- Reference
- Schellenberg MJ, Perera L, Strom CN, Waters CA, Monian B, Appel CD, Vilas CK, Williams JG, Ramsden DA, Williams RS (2016): "Reversal of DNA damage induced Topoisomerase 2 DNA-protein crosslinks by Tdp2." Nucleic Acids Res., 44, 3829-3844. doi: 10.1093/nar/gkw228.
- Abstract
- Mammalian Tyrosyl-DNA phosphodiesterase 2 (Tdp2) reverses Topoisomerase 2 (Top2) DNA-protein crosslinks triggered by Top2 engagement of DNA damage or poisoning by anticancer drugs. Tdp2 deficiencies are linked to neurological disease and cellular sensitivity to Top2 poisons. Herein, we report X-ray crystal structures of ligand-free Tdp2 and Tdp2-DNA complexes with alkylated and abasic DNA that unveil a dynamic Tdp2 active site lid and deep substrate binding trench well-suited for engaging the diverse DNA damage triggers of abortive Top2 reactions. Modeling of a proposed Tdp2 reaction coordinate, combined with mutagenesis and biochemical studies support a single Mg(2+)-ion mechanism assisted by a phosphotyrosyl-arginine cation-π interface. We further identify a Tdp2 active site SNP that ablates Tdp2 Mg(2+) binding and catalytic activity, impairs Tdp2 mediated NHEJ of tyrosine blocked termini, and renders cells sensitive to the anticancer agent etoposide. Collectively, our results provide a structural mechanism for Tdp2 engagement of heterogeneous DNA damage that causes Top2 poisoning, and indicate that evaluation of Tdp2 status may be an important personalized medicine biomarker informing on individual sensitivities to chemotherapeutic Top2 poisons.